Porphyrin-functionalized oligo- and polythiophenes†
Abstract
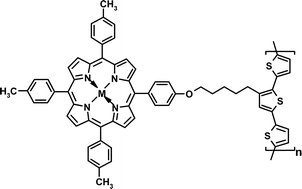
The synthesis and properties of a series of bi- and terthiophenes 3–8 substituted with meso-tetraphenylporphyrin (TPP) groups via an isolating oxaalkyl chain is described. Electrooxidative polymerization leads to the corresponding metal complexed porphyrin-functionalized polythiophenes P4–P8. The electrochemical and spectroscopic properties of the polymer films reveal the superimposition of the electronic properties of the individual π-systems. Spectroelectrochemical experiments and conductivity measurements point to a mixed charge transport mechanism. Polaronic and bipolaronic delocalization on the conjugated chains combined with electron hopping processes via the porphyrin redox centers result in high stability of the polymers against overoxidation. Importantly, hybrid materials have been obtained which at the same time exhibit properties of a conducting polymer and a redox polymer that can be used for the detection of polychlorinated phenols in amperometric sensors.

 Please wait while we load your content...
Please wait while we load your content...