Matrix infrared spectra and density functional calculations of transition metal hydrides and dihydrogen complexes
Abstract
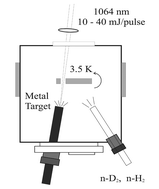
Metal hydrides are of considerable importance in chemical synthesis as intermediates in catalytic hydrogenation reactions. Transition metal atoms react with dihydrogen to produce metal dihydrides or dihydrogen complexes and these may be trapped in solid matrix samples for infrared spectroscopic study. The MH2 or M(H2) molecules so formed react further to form higher MH4, (H2)MH2, or M(H2)2, and MH6, (H2)2MH2, or M(H2)3 hydrides or complexes depending on the metal. In this critical review these transition metal and dihydrogen reaction products are surveyed for Groups 3 though 12 and the contrasting behaviour in Groups 6 and 10 is discussed. Minimum energy structures and vibrational frequencies predicted by Density Functional Theory agree with the experimental results, strongly supporting the identification of novel binary transition metal hydride species, which the matrix-isolation method is well-suited to investigate. 104 references are cited.

 Please wait while we load your content...
Please wait while we load your content...