Asymmetric synthesis of 2-alkyl- and 2-aryl-3-aminopropionic acids (β2-amino acids) from (S)-N-acryloyl-5,5-dimethyloxazolidin-2-one SuperQuat derivatives
Abstract
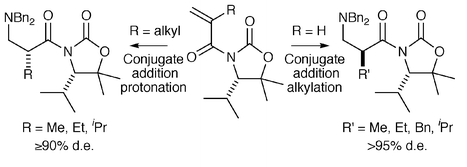
Conjugate addition of lithium amides to (S)-N-acryloyl- or (S)-N-2′-alkylacryloyloxazolidinones and alkylation or protonation of the resulting enolates with 2-pyridone respectively provides a highly stereoselective and product complementary route to a range of (R)- and (S)-2-alkyl-3-aminopropionic acids in good yield and in high ee.

 Please wait while we load your content...
Please wait while we load your content...