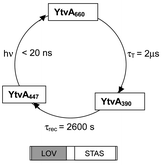
YtvA is a bacterial flavo-protein related to plant phototropin. The photochemistry of YtvA and of its isolated LOV domain (YtvA-LOV) has been characterized by optical, mass spectrometric and photocalorimetric methods. The energy content (E390) of the FMN-C4a-thiol photoadduct (YtvA390 and YtvA-LOV390) and its structural volume change (ΔV390), with respect to the parent state, have been determined by means of Laser Induced Optoacoustic Spectroscopy (LIOAS). The high value of E390, 136 and 115 kJ mol−1, respectively, ensures a large driving force for the dark recovery to the unphotolyzed state and points to a strained conformation of the protein or/and the chromophore in the photoadduct. The value of ΔV390 is significantly different for the two proteins, ΔV390
=
−12.5 ml mol−1 in YtvA and −17.2 ml mol−1 in YtvA-LOV. The kinetics of the dark recovery reaction for YtvA-LOV is slower than for full-length YtvA, with τrec
= 3900 and 2600 s at 25 °C, respectively, and shows a different temperature dependence. A similarly slow kinetics can be induced in YtvA by high ionic strength. Minor differences are observed in the fluorescence and photoadduct formation quantum yield. The overall stability is higher for YtvA than for YtvA-LOV. The data as a whole are indicative of an interaction between the two domains of YtvA, most probably mediated by electrostatic interactions that renders the full-length protein a compact and more rigid unit. The results reported here support the idea that the formation of the photoadduct changes the dynamics of the protein, depending on the conformational flexibility of the parent state. Flashing of the photoadduct induces a negligible ΔV, with 96% of the excitation energy dissipated as heat in <20 ns, indicating that the photoadduct does not undergo a photocycle on the LIOAS time scale, or that the photoinduced reactions occur with very low yield.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...