Photolysis of hop-derived trans-iso-α-acids and trans-tetrahydroiso-α-acids: product identification in relation to the lightstruck flavour of beer†
Abstract
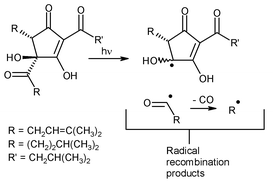
The photolysis of hop-derived trans-iso-α-acids (2a–c; naturally occurring bitter compounds present in beer) and of trans-tetrahydroiso-α-acids (5a–c; semi-synthetic advanced hop products) was investigated at 300 nm in
- This article is part of the themed collection: In honour of Jean Kossanyi

 Please wait while we load your content...
Please wait while we load your content...