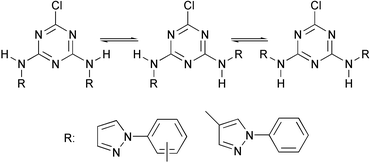
A series of 2-chloro-4,6-bis(pyrazolylamino)-1,3,5-triazines with applications in crystal engineering have been prepared. At low temperature, the presence of two or three isomers has been detected and these assigned to 4,6-diamino-1,3,5-triazine structures on the basis of comparison with model compounds. 2D-Exchange spectroscopy studies in various solvents and at different temperatures have been used to determine the equilibrium constants and the activation free energies of the restricted rotation about the amino–triazine bond. A plot of the activation free energy versus temperature showed a good linear correlation and confirmed that the same process is present in all of the compounds under investigation. Comparison with model compounds also confirmed both the occurrence of the restricted rotation and the 4,6-diamino-1,3,5-triazine tautomerism for triazines 1–4 in solution. The structure of compound 1 has been determined in the solid state by X-ray crystallography and consists of a 4,6-diamino-1,3,5-triazine structure stabilized by intra and intermolecular hydrogen bonds.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...