A tricycloheptane product in cationic rearrangements
Abstract
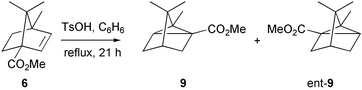
The bicycloheptene 6 rearranged in acid to give the tricycloheptane 9, as shown by an X-ray crystal structure determination of the p-nitrobenzoate 10 derived from it. Earlier results in the literature had already indicated that this isomer was the thermodynamic sink. This apparently crowded structure, with its three contiguous quaternary centres, is, nevertheless, lower in energy than other accessible but less crowded structures, because of electronic stabilisation of the more substituted

 Please wait while we load your content...
Please wait while we load your content...