Preparation of enantiomerically pure pyridyl amino acids from serine
Abstract
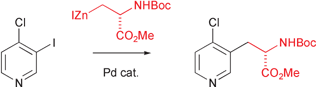
A range of substituted pyridyl amino acids have been prepared by palladium catalysed cross-coupling of serine-derived organozinc reagents with differently substituted halopyridines. Following this procedure a DMAP analogue has been synthesised and used as a building block in the preparation of two related

 Please wait while we load your content...
Please wait while we load your content...