Bend ribbon-forming tetrahydrofuran amino acids†
Abstract
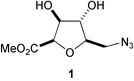
Short oligomeric chains of C-glycosyl β-D-arabinofuranose configured tetrahydrofuran amino acids (where the C-2 and C-5 substituents of the tetrahydrofuran ring are cis to each other) exhibit a well-defined repeating turn secondary structure stabilised by (i, i − 2) inter-residue hydrogen bonds. This is in contrast to the epimeric α-D-arabinofuranose oligomer (where the C-2 and C-5 substituents of the tetrahydrofuran ring are trans to each other) in which there is no indication of any secondary structure in solution.

 Please wait while we load your content...
Please wait while we load your content...