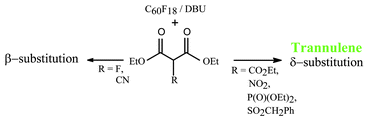
A range of tertiary carbanions XCH(CO2Et)2 of differing sizes have been reacted with C60F18 to assess the steric effect of X on the position of nucleophilic substitution. For X
= CO2Et, NO2, P(O)(OMe)2, SO2CH2Ph, the all trans annulenes (trannulenes) were obtained as a result of extended SN2′
(i.e. SN2″) substitution; in the case of the phosphorus compound, with reduced amounts of base (DBU) dephosphonylation of one or more P(O)(OMe)2 groups by hydrogen occurred. Trannulene formation did not occur for X
= F, CN due to the smaller size of the nucleophile, and in the latter case substitution was shown to take place by an SN2′ mechanism, resulting in the addend being adjacent to a fluorine addend. Trannulenes (X
= CO2Et, Br, Cl) exhibited reversible one-electron reductions at potentials (−0.02 to −0.09 V) significantly more positive than for [60]fullerene. Trannulene (X
= NO2) exhibited an irreversible one-electron reduction (0.08 V); the irreversibility may be associated with fluorine loss. Conformational isomerism at temperatures below 298 K was observed for all trannulene derivatives as a result of eclipsing addend–addend interactions. Minimum energy conformations with a rotational energy barrier of 12–15 kcal mol−1 were observed when these interactions are calculated using molecular mechanics.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...