A2-Rhodopsin: a new fluorophore isolated from photoreceptor outer segments
Abstract
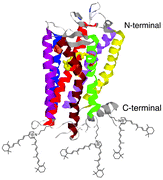
A2E and iso-A2E are fluorescent amphiphilic pyridinium bisretinoids involved in age-related macular degeneration (AMD). It is now shown that the presence of high exogenous concentrations of

 Please wait while we load your content...
Please wait while we load your content...