NMR analysis of lignins in CAD-deficient plants. Part 1. Incorporation of hydroxycinnamaldehydes and hydroxybenzaldehydes into lignins
Abstract
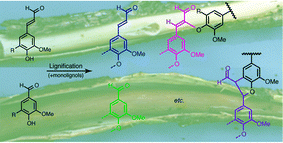
Peroxidase/H2O2-mediated radical coupling of 4-hydroxycinnamaldehydes produces 8–O–4-, 8–5-, and 8–8-coupled dehydrodimers as has been documented earlier, as well as the 5–5-coupled dehydrodimer. The 8–5-dehydrodimer is however produced kinetically in its cyclic phenylcoumaran form at neutral pH. Synthetic

 Please wait while we load your content...
Please wait while we load your content...