Isoprenoid biosynthesis via the MEP pathway. Synthesis of (3R,4S)-3,4-dihydroxy-5-oxohexylphosphonic acid, an isosteric analogue of 1-deoxy-d-xylulose 5-phosphate, the substrate of the 1-deoxy-d-xylulose 5-phosphate reducto-isomerase
Abstract
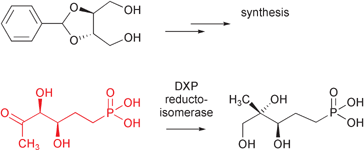
(3R,4S)-3,4-Dihydroxy-5-oxohexylphosphonic acid, an isosteric analogue of 1-deoxy-D-xylulose 5-phosphate (DXP), was obtained in enantiomerically pure form from (+)-2,3-O-benzylidene-D-threitol by a seven-step sequence. This phosphonate did not affect the growth of Escherichiacoli. It did not inhibit the 1-deoxy-D-xylulose 5-phosphate reductoisomerase (DXR), but was converted by this enzyme into (3R,4R)-3,4,5-trihydroxy-3-methylpentylphosphonic acid, an isosteric analogue of 2-C-methyl-D-erythritol 4-phosphate. The enzyme was, however, less efficient with the methylene phosphonate analogue than with the natural substrate.

 Please wait while we load your content...
Please wait while we load your content...