Pyrazole-4-sulfonate networks of alkali and alkaline-earth metals. Effect of cation size, charge, H-bonding and aromatic interactions on the three-dimensional supramolecular architecture
Abstract
Variation of ![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , a = 5.9972(6)
Å, b = 9.2102(9)
Å, c = 12.957(1)
Å, α = 76.536(2)°, β = 89.264(2)°, γ = 79.057(2)°, V = 683.0(1)
Å, Z = 2; BaL(H2O)·H2O, monoclinic P21/c, a = 11.386(1)
Å, b = 4.9098(6)
Å, c = 24.348(3)
Å, β = 90.702(2)°, V = 1361.0(3)
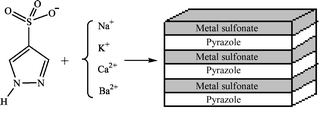
Å, Z = 4. All four metal sulfonate networks have an alternating inorganic–organic layered structure, reinforced by multiple H-bonding within the inorganic layer as well as between the organic and inorganic layers; extended π–π stacking and edge-to-face aromatic interactions within the organic layer provide further lattice strength.
, a = 5.9972(6)
Å, b = 9.2102(9)
Å, c = 12.957(1)
Å, α = 76.536(2)°, β = 89.264(2)°, γ = 79.057(2)°, V = 683.0(1)
Å, Z = 2; BaL(H2O)·H2O, monoclinic P21/c, a = 11.386(1)
Å, b = 4.9098(6)
Å, c = 24.348(3)
Å, β = 90.702(2)°, V = 1361.0(3)
Å, Z = 4. All four metal sulfonate networks have an alternating inorganic–organic layered structure, reinforced by multiple H-bonding within the inorganic layer as well as between the organic and inorganic layers; extended π–π stacking and edge-to-face aromatic interactions within the organic layer provide further lattice strength.

 Please wait while we load your content...
Please wait while we load your content...