Synthesis of dimethyl carbonate and glycols from carbon dioxide, epoxides and methanol using heterogeneous Mg containing smectite catalysts: effect of reaction variables on activity and selectivity performance
Abstract
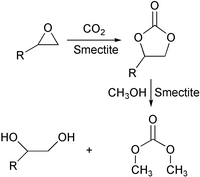
This paper reports the effect of various reaction variables on the activity and selectivity performance on a two-step synthesis of dimethyl carbonate (DMC) and glycol from propylene oxide, carbon dioxide and methanol using a heterogeneous Mg containing smectite catalyst. The first step, the reaction of propylene oxide with CO2 to form propylene carbonate, and the second step, the transesterification reaction of the cyclic carbonate such as ethylene carbonate with methanol to DMC and ethylene glycol, have been studied. The catalyst was found to be effective for one-pot synthesis of DMC, i.e. the sequential reaction of the epoxide, CO2 and methanol.

 Please wait while we load your content...
Please wait while we load your content...