The chemistry and biochemistry of the copper–radical interaction
Abstract
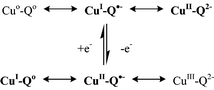
This Perspective describes the plausible oxidation state combinations of Cun/Lm systems where Lm is an O- or N-based redox system with a paramagnetic intermediate such as O2˙−, NO˙, phenoxyl, o-semiquinone or azo radical anion. The biochemical relevance is discussed using superoxide dismutase, nitrite reductase, galactose oxidase and amine oxidase enzyme examples. Particular emphasis will be given to the identification of oxidation states and to the manifestations of intramolecular electron transfer in enzymes and model compounds.

 Please wait while we load your content...
Please wait while we load your content...