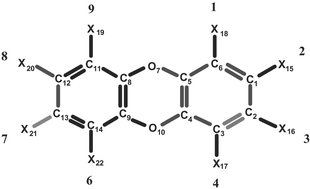
Quantum topological molecular similarity. Part 5. Further development with an application to the toxicity of polychlorinated dibenzo-p-dioxins†(PCDDs)
Abstract
A new method called quantum topological molecular similarity (QTMS), which was previously introduced, is further developed and applied. An excellent and statistically validated QSAR is obtained for the Hammett acidity constants of a set of 68

 Please wait while we load your content...
Please wait while we load your content...