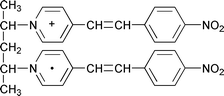
Steady state photolysis of tetraphenylborate salts of meso-2,4-bis(4-(4-nitrostyryl)pyridiniumyl)pentane and 1,3-bis(4-(4-nitrostyryl)pyridiniumyl)propane has been studied by absorption and electron paramagnetic resonance (EPR) spectroscopy in solution at room temperature. The absorption spectra of these compounds upon irradiation at 405 nm in an inert atmosphere showed a strong peak in the visible region and two broad peaks in the near infrared (NIR) region. The meso-2,4-pentane derivative caused stronger electronic interaction between a photogenerated styrylpyridinyl radical and a parent styrylpyridinium cation, and hence showed two slightly blue shifted NIR bands around 920 and 1734 nm compared with those in the 1,3-propane derivative. During storage in the dark, the NIR absorption peak at longer wavelength gradually changed its shape and showed a new band at 1559 nm, which increased until 20 h and was maintained over 100 h. The nature of electronic interactions
responsible for such specific NIR bands was studied in detail spectroscopically. EPR spectra clearly indicated that the unpaired electron was not shared completely between two chromophores as in a normal dimer radical cation but was exchanged rather rapidly between them, probably due to the conformational confinement at one end of these molecules.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...