Nitroalkanes as nucleophiles in a self-catalytic Michael reaction
Abstract
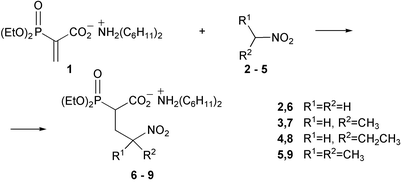
A simple, effective procedure for the preparation of 4-nitroalkanoates 6–9 by the Michael reaction of nitroalkanes 2–5 with the acrylate 1 is described. The primary nitro adduct 6 undergoes isomerization to hydroxamic acid 10 while heated in boiling nitromethane. Consecutive reactions of the latter compound lead to the formation of N-hydroxysuccinimide 11 and its N-ethoxy derivative 12. The spontaneous Nef reaction of the mother 4-nitrobutanoic acid 15 gives N-hydroxysuccinimide 14. The analogous reaction of secondary nitroalkanoic acids 16 and 17 provides 4-oxoalkanoic acids 18 and 19, respectively. Intramolecular participation by the carboxylic acid group in the Nef reaction is proposed.

 Please wait while we load your content...
Please wait while we load your content...