Making conjugated connections to porphyrins: a comparison of alkyne, alkene, imine and azo links
Abstract
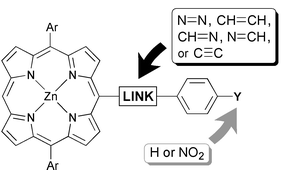
A series of porphyrins 5–9 has been prepared, in which an aryl substituent is linked to the porphyrin via azo, imine, alkene and alkyne bridges. The strength of aryl–porphyrin electronic coupling in these systems was evaluated from the red shift and intensification of the Q band absorption and emission spectra, and from the incremental red shift on changing from the phenyl to a 4-nitrophenyl substituent. The azo link provides the strongest electronic communication between the porphyrin and the benzene ring. The crystal structures of azo compounds 5a and 5c show that the porphyrin and benzene rings are almost coplanar, whereas imine 7a and alkene 8a are significantly twisted in the solid state. Imine and alkyne linked porphyrin dimers 18 and 23 were also synthesized; the alkyne-linked dimer is much more conjugated than its imine-linked analogue.

 Please wait while we load your content...
Please wait while we load your content...