Synthesis of perylene–porphyrin building blocks and rod-like oligomers for light-harvesting applications†
Abstract
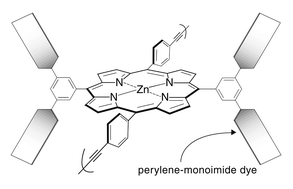
We present the synthesis of four perylene–porphyrin building blocks for use in Glaser, Sonogashira, or Suzuki polymerizations. The building blocks bear synthetic handles (4-ethynylphenyl, 4-iodophenyl, bromo) at the trans (5,15) meso-positions of a zinc porphyrin and contain two or four perylene-monoimide dyes attached at the 3,5-positions of the non-linking meso-aryl rings of the porphyrin. Each perylene-monoimide bears three 4-tert-butylphenoxy substituents (at the 1-, 6-, and 9-positions) and two isopropyl groups (on the N-aryl unit) for increased solubility. In each case the intervening linker is a diarylethyne unit that bridges the N-imide position of the perylene and the meso-position of the porphyrin. The perylene–porphyrin building blocks were prepared by (1) reaction of a diperylene-dipyrromethane with an aldehyde yielding a trans-A2B2-porphyrin, (2) reaction of a diperylene-aldehyde with a dipyrromethane yielding a trans-A2B2-porphyrin, and (3) reaction of a diperylene-dipyrromethane with a dipyrromethane-dicarbinol yielding a trans-AB2C-porphyrin or ABCD-porphyrin. The building blocks were subjected to Glaser, Sonogashira, or Suzuki coupling conditions in an effort to prepare oligomers containing porphyrins joined via 4,4′-diphenylbutadiyne (dpb), 4,4′-diphenylethyne (dpe), or 1,4-phenylene linkers (p), respectively. Each porphyrin in the backbone bears two or four pendant perylene-monoimide dyes. The Glaser and Sonogashira reactions afforded a distribution of oligomers, whereas the Suzuki reaction was unsuccessful. The oligomers were soluble in solvents such as toluene, THF, or CHCl3 enabling routine handling. The use of perylenes results in (1) increased light-harvesting efficiency particularly in the green spectral region where porphyrins are relatively transparent and (2) greater solubility than is achieved with the use of porphyrins alone. The soluble perylene–porphyrin oligomers are attractive for use as light-harvesting materials in molecular-based solar cells.

 Please wait while we load your content...
Please wait while we load your content...