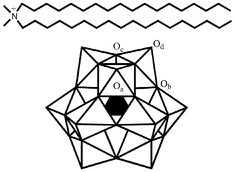
A highly ordered multilayer film was formed by casting chloroform solutions of an ionic complex between a dimethyldioctadecylammonium (DODA) and a phosphomolybdate anion PMo12O403−
(PMo12) on a glassy carbon electrode and solid substrates (CaF2, quartz, and borosilicate glass). A well-ordered lamellar superlattice structure was identified by X-ray diffraction (XRD) and differential scanning calorimetry (DSC) experiments. IR spectra showed that the Keggin structure of the phosphomolybdate anion is preserved in the composite film. The superlattice film showed good photochromic and electrochemical properties. Irradiated with UV light, the transparent film changed from colorless to blue. Then, bleaching occurred when the film was in contact with ambient air or O2 in the dark. A possible photochromic mechanism was proposed according to electron paramagnetic resonance (EPR),
Fourier transform infrared spectra (FTIR), UV–vis spectra, and related literature. The electrochemical behavior of the composite film was also examined by cyclic voltammetry in detail.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...