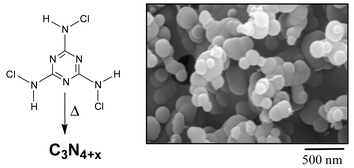
There is increasing interest in high surface area carbon nitride materials as potential coordinatively active analogs of amorphous carbon systems. It is generally difficult to produce extended carbon structures with high nitrogen contents. This article describes a facile molecular decomposition process that produces bulk quantities of an amorphous nitrogen-rich carbon nitride material, C3N4+x where 0.5 < x < 0.8, in only a few seconds without the use of complex experimental apparatus. The trichloromelamine molecular precursor [(C3N3)(NHCl)3] rapidly decomposes when heated externally above 185 °C or when brought into contact with a heated filament. Morphological studies show that the rapid synthesis process produces a porous, sponge-like material containing spherical nanofeatures (<300 nm). These amorphous carbon nitrides were analyzed by IR, NMR,
optical, and X-ray photoelectron spectroscopy, which indicate that the carbon centers have primarily sp2 hybridization, triazine (C3N3) rings are retained in the product, and nitrogen species bridge triazines. These C3N4+x materials are also luminescent in the blue region even after annealing to 400 °C. They exhibit thermal and chemical stability with no significant decomposition until 600 °C or reactivity with concentrated aqueous base.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...