Abstract
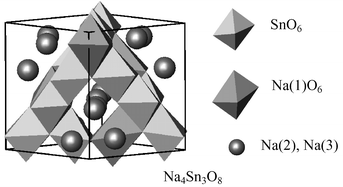
A new ternary Na–Sn–O compound, Na4Sn3O8, has been synthesized from a mixture of Na2CO3 and SnO2 by heating at 1270–1400 °C for 6–24 h in air using a powder bed. The crystal structure of Na4Sn3O8 has been refined by the Rietveld analysis of the powder X-ray diffraction data. Na4Sn3O8 crystallizes in the cubic system (a = 9.1815(2) Å, Z = 4) with the space group of either P4332 or P4132. The unit cell contains twelve SnO6 and four NaO6 octahedra connecting by edge-sharing and additionally twelve sodium atoms distributed in octahedral holes.

 Please wait while we load your content...
Please wait while we load your content...