Efficient, halide free synthesis of new, low cost ionic liquids: 1,3-dialkylimidazolium salts containing methyl- and ethyl-sulfate anions
Abstract
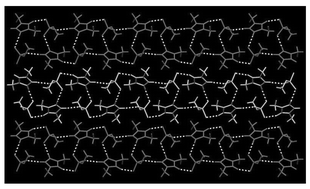
New low-cost ionic liquids containing methyl- and ethyl-sulfate anions can be easily and efficiently prepared under ambient conditions by the reaction of 1-alkylimidazoles with dimethyl sulfate and diethyl sulfate. The preparation and characterization of a series of 1,3-dialkylimidazolium alkyl sulfate and 1,2,3-trialkylimidazolium alkyl sulfate salts are reported. 1,3-Dialkylimidazolium salts containing at least one non-methyl N-alkyl substituent are liquids at, or below room, temperature. Three salts were crystalline at room temperature, the single crystal X-ray structure of 1,3-dimethylimidazolium methyl sulfate was determined and shows the formation of discrete ribbons comprising of two anion–cation hydrogen-bonded chains linked via intra-chain hydrogen-bonding, but little, or no inter-ribbon hydrogen-bonding. The salts are stable, water soluble, inherently ‘chloride-free’, display an electrochemical window of greater than 4 V, and can be used as alternatives to the corresponding halide salts in metathesis reactions to prepare other ionic liquids including 1-butyl-3-methylimidazolium hexafluorophosphate.

 Please wait while we load your content...
Please wait while we load your content...