Selective oxidation of cyclohexane in compressed CO2 and in liquid solvents over MnAPO-5 molecular sieve
Abstract
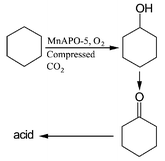
The selective oxidation reaction of cyclohexane with MnAPO-5 molecular sieve catalyst to produce cyclohexanol and cyclohexanone was carried out in compressed CO2 at 398.2 K using oxygen as an oxidant, and the reaction mixture was controlled to be in the two-phase region, very close to the critical point, and in the supercritical (sc) region of the reaction system. The effect of a small amount of butyric acid cosolvent on the reaction in scCO2 was also studied. The reaction was also conducted in liquid butyric acid, benzene and CCl4, and in the absence of any solvent. It has been observed that the conversion and selectivity of the reaction in compressed CO2 change considerably with the phase behavior or the apparent density of the reaction system. Addition of a small amount (0.2 mol%) of butyric acid cosolvent to scCO2 enhances the conversion significantly, and the selectivity also changes considerably. The by-products of the reaction in compressed CO2 with and without the cosolvent are much less than that of the reaction in liquid solvents or in the absence of solvents. It is advantageous to conduct the reaction in supercritical CO2.

 Please wait while we load your content...
Please wait while we load your content...