Synthesis, characterisation and catalytic activity of metal complexes of neutral, unsymmetrical P/S ferrocenediyl ligands
Abstract
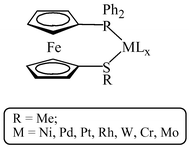
Two asymmetric 1,1′-disubstituted ferrocenediyl ligands, 1-(diphenylphosphino)-1′-(methylthio)ferrocene (1) and 1-(diphenylphosphino)-1′-(mesitylthio)ferrocene (2) featuring phosphine and thioether substituents have been conveniently synthesised and the coordination chemistry of 1 probed by reaction with transition metal reagents. With Group 10 metal and Rh(I) species, chelating complexes are formed in high yield and a monodentate bis ligand complex with trans phosphorus ligation can also be synthesised using a Pd(II) species and three equivalents of 1. With [M(CO)5thf] (M = Cr, Mo or W), 1 forms a mixture of monodentate, P-bound pentacarbonyl and P/S-chelating tetracarbonyl products. The monodentate pentacarbonyl product can be converted into the chelating tetracarbonyl species via prolonged reflux in toluene. Preliminary studies show that 1, in combination with Pd(0) precursors, can act as a catalyst for the Suzuki coupling reaction.

 Please wait while we load your content...
Please wait while we load your content...