Mono- and di-nuclear complexes of the ligands 3,4-di(2-pyridyl)-1,2,5-oxadiazole and 3,4-di(2-pyridyl)-1,2,5-thiadiazole; new bridges allowing unusually strong metal–metal interactions
Abstract
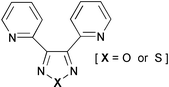
The syntheses of two new ligands, 3,4-di(2-pyridyl)-1,2,5-oxadiazole (dpo) and 3,4-di(2-pyridyl)-1,2,5-thiadiazole (dpt), are described. Complexes with palladium and copper have seven-membered chelate rings with coordination through the two pyridine nitrogens, whereas in the silver nitrate complex of dpt the ligand acts as a bridge between metal centres. Studies of the mononuclear ruthenium complexes indicate five-membered chelate rings (involving donor nitrogen atoms from each of a pyridine ring and the oxadiazole or thiadiazole ring) and reveal that these ligands are very electron deficient and possess very low energy π* orbitals. Dinuclear ruthenium complexes have been prepared and the diastereoisomers separated and crystallographically characterised. Electrochemical studies of these complexes reveal remarkably strong metal–metal interactions, which also depend on the stereoisomeric form. Some heterodinuclear complexes have also been prepared.

 Please wait while we load your content...
Please wait while we load your content...