Characterisation of aqueous peroxomolybdate catalysts applicable to pulp bleaching†
Abstract
The equilibrium speciation in the system pH+
+
qMoO42−
+
rH2O2
+
sSO42−
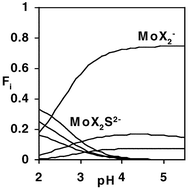
⇌ (H+)p(MoO42−)q(H2O2)r(SO42−)s in 0.300 M Na2(SO4) medium at 25 °C has been determined from potentiometric titration data in the ranges 2.0 ≤ pH ≤ 5.5, 5.00 ≤ [Mo]tot/mM ≤ 80.00, 0 ≤ [H2O2]tot/mM ≤ 245 and 273.07 ≤ [SO42−]tot/mM ≤ 320.20. Species with the following compositions were found: MoX− (1,1,1,0), MoX (2,1,1,0), MoX2− (1,1,2,0), MoX2 (2,1,2,0), MoX2S2−
(2,1,2,1), Mo2X42− (2,2,4,0), Mo7X6− (8,7,1,0), Mo7X5− (9,7,1,0), Mo7X4− (10,7,1,0) and Mo7X3− (11,7,1,0). The numbers in parentheses refer to the values of p, q, r and s in the formula above. The numbers and charges of molybdenum (Mo),

 Please wait while we load your content...
Please wait while we load your content...