Abstract
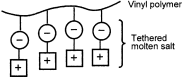
We synthesized a series of imidazolium cations containing covalently-bound anionic sites, such as sulfonate or sulfonamide groups. These zwitterionic imidazolium salts were found to form molten salts just like ordinary imidazolium salts. However, regardless of the high ion density, these ions cannot migrate along potential gradients induced in the bulk. This is a new and unique characteristic in molten salts. When other salts were added to this, the ions generated from the newly added salts were able to behave as carrier ions. The ionic conductivity of a pure molten salt was 10−9 S cm−1 at 25 °C, but jumped to 10−5 S cm−1 by adding an equimolar amount of lithium bis(trifluoromethanesulfonyl)imide (LiTFSI) at 50 °C. The zwitterionic salt having a sulfonamide group instead of sulfonate had an ionic conductivity of 10−4 S cm−1 at 50 °C after adding an equimolar amount of LiTFSI. These zwitterionic imidazolium salts having vinyl groups were synthesized and polymerized. In spite of their rubber-like properties they showed excellent ionic conductivities of around 10−5 S cm−1 at 50 °C following the addition of an equimolar amount of LiTFSI to the imidazolium cation unit.

 Please wait while we load your content...
Please wait while we load your content...