Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
EDOT–diketopyrrolopyrrole copolymers for polymer solar cells†
Chao
Wang
a,
Christian J.
Mueller
b,
Eliot
Gann
ac,
Amelia C. Y.
Liu
d,
Mukundan
Thelakkat
b and
Christopher R.
McNeill
*a
aDepartment of Materials Science and Engineering, Monash University, Wellington Road, Clayton, VIC 3800, Australia. E-mail: christopher.mcneill@monash.edu
bApplied Functional Polymers, Macromolecular Chemistry I, University of Bayreuth, 95440 Bayreuth, Germany
cAustralian Synchrotron, 800 Blackburn Road, Clayton, VIC 3168, Australia
dMonash Centre for Electron Microscopy and School of Physics, Monash University, Clayton, Victoria 3800, Australia
First published on 3rd February 2016
Abstract
The photovoltaic properties of a series of diketopyrrolo[3,4-c]pyrrole (DPP) copolymers containing 3,4-ethylenedioxythiophene (EDOT) as a comonomer are reported. With use of different aryl flanking units on the DPP core, namely thiophene, pyridine or phenyl, optical gaps ranging from 1.91 eV to 1.13 eV are achieved. When blended with the fullerene derivative [6,6]-phenyl C71-butyric acid methyl ester (PC71BM), the thiophene-flanked copolymer PDPP[T]2-EDOT with an optical gap of 1.13 eV was found to have the best photovoltaic performance, with an efficiency of 2.5% in an inverted device architecture. Despite having the lowest open circuit voltage of the three polymers studied, PDPP[T]2-EDOT-based devices were able to achieve superior efficiencies due to the high short circuit current of up to ∼15 mA cm−2. PDPP[T]2-EDOT-based devices also exhibit higher external quantum efficiencies which are associated with a superior microstructure – as revealed by transmission electron microscopy (TEM) and grazing incidence wide-angle X-ray scattering (GIWAXS) – which is associated with the enhanced aggregation tendency of PDPP[T]2-EDOT chains. In particular PDPP[T]2-EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM blends were found to have a finer phase separated morphology with superior thin-film crystallinity. Surface morphology was also investigated with atomic force microscopy and near-edge X-ray absorption fine-structure spectroscopy.
PC71BM blends were found to have a finer phase separated morphology with superior thin-film crystallinity. Surface morphology was also investigated with atomic force microscopy and near-edge X-ray absorption fine-structure spectroscopy.
(A) Introduction
Harnessing solar energy is a promising way to meet the world's rapidly increasing energy demand. Intensive research has been conducted on organic photovoltaic technologies (OPV) over the past two decades since the first demonstration of a donor/acceptor bilayer planar heterojunction in 1979.1,2 OPVs offer several advantages compared to conventional solar technologies including solution processability, low cost, flexible substrates, semitransparency and ease of fabrication. The bilayer heterojunction architecture has certain limitations including a limited donor–acceptor interfacial area and the requirement of long exciton diffusion lengths in order to ensure sufficient exciton dissociation. Excitons in most organic semiconductors have very short lifetimes with associated diffusion lengths of about 4–20 nm,3,4 and thus only excitons within an exciton diffusion length of the donor–acceptor interface are able to dissociate into free charges before recombining. The bulk heterojunction (BHJ) architecture was a breakthrough for the organic photovoltaics field, greatly enhancing the yield of dissociated excitons through the forming of a nano-scale donor–acceptor morphology that evolves during the spin coating of a blend solution.5–7 Through the development of techniques to control morphology either during or post film deposition and the development of high materials with improved light absorption and charge transporting properties, OPV devices have achieved efficiencies above 10%.8–10The development of OPV technology has gone hand-in-hand with the innovation of new materials. The power conversion efficiency of organic solar cells is a product of open-circuit voltage (VOC), short-circuit current (JSC) and fill factor (FF). The highest occupied molecular orbital (HOMO) level of the electron donor material and the lowest unoccupied molecular orbital (LUMO) level of the electron acceptor material roughly determine the maximum achievable VOC, while the lowest optical gap (Eg) of the two materials largely determines maximum possible JSC.11 With regard to polymer donors, the key issues of polymer design include engineering the band gap and energy levels to achieve high JSC and VOC. In an ideal case, a low band-gap polymer, whose absorption extends into the inferred region, can greatly improve the JSC as more photons can be converted to electrons. Thus fine tuning of HOMO and LUMO levels is required to achieve a high VOC with a small band gap, while maintaining a LUMO level high enough for efficient charge separation.12 In addition, high performance polymeric donor materials should possess high hole mobilities and the ability to be processed with a suitable acceptor to form an optimal phase-separated morphology.
The recent success of polymer/fullerene solar cells that utilise a polymeric donor and fullerene acceptor has resulted largely from the development of new low band-gap donor materials. The donor–acceptor design, where electron rich and electron deficient units alternate along the copolymer backbone is commonly used to tune the HOMO and LUMO energy levels and the optical gap of these polymers.13 While many different building blocks have been utilised for the construction of donor–acceptor copolymers, the electron deficient diketopyrrolopyrrole (DPP) unit has proved to be a versatile acceptor unit with DPP-based copolymers possessing low band gaps for polymer solar cell applications14,15 and high carrier mobilities in field-effect transistors.16 In particular power conversion efficiencies of up to 9.4% have been achieved,17–19 with bandgaps as low as 1.13 eV also realised for DPP-based polymers.20 It is the strong electron deficient character of the DPP moiety, combined with high planarity and aggregation, that allows these novel materials to absorb in the near infrared region and exhibit good electron and hole mobilities.16
Here we report the photovoltaic properties of novel DPP-based copolymers that incorporate the electron rich 3,4-ethylenedioxythiophene (EDOT) as comonomer with different aryl flanking units on either side of the DPP core, see Fig. 1.21 The use of EDOT as a comonomer is motivated by its strong electron rich character which can enhance the p-type character of PDPP materials and provide non-covalent, diffusive H⋯O interactions to the adjacent aryl units in the backbone.19 Through the use of different flanking units, tuning of the bandgap is achieved with an optical bandgap as low as 1.13 eV achieved with the use of thiophene flanking units which promote a more coplanar backbone. Interestingly, the highest efficiencies are achieved with the lowest bandgap material that exhibits a broad spectral response from 350 nm to over 1000 nm. As well as characterising the performance of these novel polymers in inverted and standard device configurations, the microstructure of blends with the fullerene derivative [6,6]-phenyl C71-butyric acid methyl ester (PC71BM) are also reported.
(B) Result and discussion
Materials
Fig. 1 presents the chemical structures of the three DPP–EDOT polymers investigated. These three polymers employ the DPP moiety as the electron deficient unit along with the EDOT unit as an electron rich comonomer. For the polymer PDPP[T]2-EDOT, thiophene units are used as flanking units either side of the DPP moiety. For the polymer PDPP[Py]2-EDOT pyridine units are used as flanking units while for PDPP[Ph]2-EDOT phenyl units are used as flanking units. The synthesis of these materials has been reported separately.21 The electrochemical and optoelectronic properties are strongly influenced by the choice of flanking unit, with the HOMO, LUMO and bandgap values of these polymers summarised in Table 1. The low band gap of the thiophene containing polymer is attributed not only to the planar structure of the polymer backbone but also to a very strong push–pull effect of the electron rich EDOT and the electron deficient DPP core.21Table 1 also summarises the molecular weights of the polymers with a number average molecular weight (Mn) of 142 kg mol−1 for PDPP[T]2-EDOT, Mn = 25.7 kg mol−1 for PDPP[Py]2-EDOT and Mn = 16.7 kg mol−1 for PDPP[Ph]2-EDOT. With variation of the choice of flanking unit, tuning of the bandgap is achieved with an optical gap (Eopt determined from absorption onset in film21) of 1.13 eV for PDPP[T]2-EDOT, Eopt = 1.59 eV for PDPP[Py]2-EDOT and Eopt = 1.91 eV for PDPP[Ph]2-EDOT.| Polymer | M n (kg mol−1) | Đ | HOMO (eV) | LUMO (eV) | E g (eV) | E opt (eV) |
|---|---|---|---|---|---|---|
| PDPP[T]2-EDOT | 142 | 2.7 | −5.73 | −3.66 | 2.07 | 1.13 |
| PDPP[Py]2-EDOT | 25.7 | 2.4 | −6.07 | −3.74 | 2.33 | 1.59 |
| PDPP[Ph]2-EDOT | 16.7 | 2.1 | −5.89 | −3.53 | 2.36 | 1.91 |
Optical properties
Fig. 2 presents the normalized optical absorption spectra of blended films of PDPP[T]2-EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM, PDPP[Py]2-EDOT
PC71BM, PDPP[Py]2-EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM and PDPP[Ph]2-EDOT
PC71BM and PDPP[Ph]2-EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM. These blends were are all spin-coated from chloroform solutions with 2 vol% of DIO additive and a weight ratio of 1
PC71BM. These blends were are all spin-coated from chloroform solutions with 2 vol% of DIO additive and a weight ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 polymer
2 polymer![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) fullerene. Both polymer and fullerene components contribute to the absorption spectrum of the blend film, with PC71BM contributing at wavelengths lower than 600 nm and a distinct peak in the visible at around 465 nm and further peaks in the UV range. The absorption onsets of the polymers vary from 650 nm for PDPP[Ph]2-EDOT to 780 nm for PDPP[Py]2-EDOT and extend out to 1100 nm for PDPP[T]2-EDOT, matching the absorption features previously seen in neat polymer films.21 The high band gap PDPP[Ph]2-EDOT has a relatively narrow absorption range with only one distinct peak at 550 nm, with the lack of vibronic features attributed to its low planarisation due to large dihedral angles and hence decreased delocalization.21 The intermediate optical gap PDPP[Py]2-EDOT
fullerene. Both polymer and fullerene components contribute to the absorption spectrum of the blend film, with PC71BM contributing at wavelengths lower than 600 nm and a distinct peak in the visible at around 465 nm and further peaks in the UV range. The absorption onsets of the polymers vary from 650 nm for PDPP[Ph]2-EDOT to 780 nm for PDPP[Py]2-EDOT and extend out to 1100 nm for PDPP[T]2-EDOT, matching the absorption features previously seen in neat polymer films.21 The high band gap PDPP[Ph]2-EDOT has a relatively narrow absorption range with only one distinct peak at 550 nm, with the lack of vibronic features attributed to its low planarisation due to large dihedral angles and hence decreased delocalization.21 The intermediate optical gap PDPP[Py]2-EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM has two distinct peaks at around 640 and 710 nm consistent with vibronic features while the low band gap PDPP[T]2-EDOT has a broad near infrared absorption band ranging from 640 nm to 1100 nm with two distinct vibronic peaks at 950 and 850 nm.
PC71BM has two distinct peaks at around 640 and 710 nm consistent with vibronic features while the low band gap PDPP[T]2-EDOT has a broad near infrared absorption band ranging from 640 nm to 1100 nm with two distinct vibronic peaks at 950 and 850 nm.
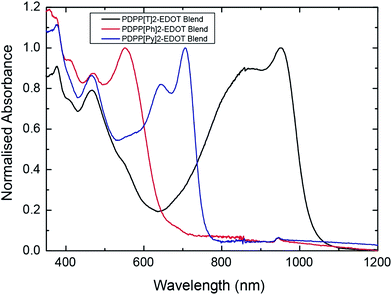 | ||
Fig. 2 Ultraviolet to near-infrared absorption spectra of blend films of PDPP[T]2-EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM, PDPP[Py]2-EDOT PC71BM, PDPP[Py]2-EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM and PDPP[Ph]2-EDOT PC71BM and PDPP[Ph]2-EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM. PC71BM. | ||
Device performance
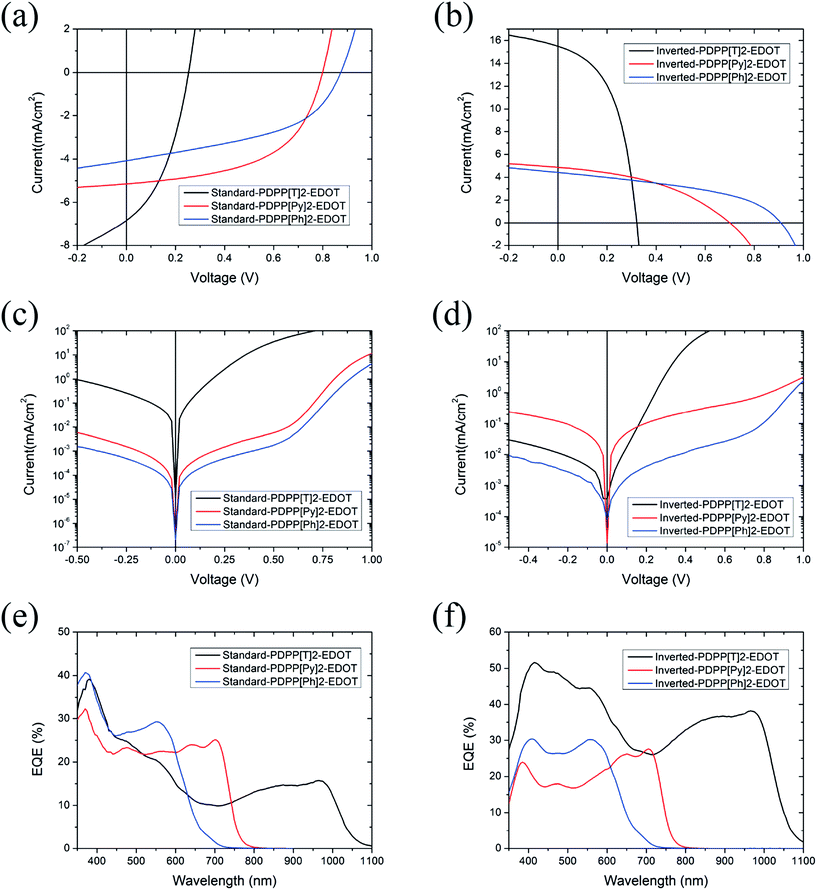
In this study, the performance of both standard and inverted devices has been evaluated. Devices with a standard structure consisted of ITO/MoO3/active layer/Ca/Al, while devices based on an inverted structure consisted of ITO/ZnO/PEIE/active layer/MoO3/Ag. The active layers are all spin-coated from chloroform solutions with 2 vol.% DIO with the active layer thickness separately optimised for each polymer. Details of device fabrication and testing are described in the Experimental section. Fig. 3a–d presents the current density versus voltage characteristics (J–V curves) of devices under AM1.5G simulated sunlight and in the dark for both standard and inverted structures. Device performance parameters such as JSC, VOC, FF and power conversion efficiency (PCE) are summarized in Table 2. In general, EDOT-polymer devices with a high band gap show a higher VOC and a lower JSC, while low band gap EDOT-polymer devices exhibit a higher JSC and a lower VOC. The standard architecture PDPP[T]2-EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM device shows an efficiency of only 0.69% with a short circuit current of 6.8 mA cm−2 and an open-circuit voltage of 0.25 V. In comparison, we found significant improvement in performance for the inverted device structure for PDPP[T]2-EDOT
PC71BM device shows an efficiency of only 0.69% with a short circuit current of 6.8 mA cm−2 and an open-circuit voltage of 0.25 V. In comparison, we found significant improvement in performance for the inverted device structure for PDPP[T]2-EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM blends with an almost 4-fold increase in efficiency to 2.5%, mainly resulting from an increase in JSC to 15.5 mA cm−2 along with improvements in VOC (an increase from 0.25 V to 0.32 V) and FF (from 0.40 to 0.50). The standard PDPP[Py]2-EDOT
PC71BM blends with an almost 4-fold increase in efficiency to 2.5%, mainly resulting from an increase in JSC to 15.5 mA cm−2 along with improvements in VOC (an increase from 0.25 V to 0.32 V) and FF (from 0.40 to 0.50). The standard PDPP[Py]2-EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM device on the other hand shows a higher PCE (2.2%), compared with inverted structure device (1.4%). The JSC of both types of PDPP[Py]2-EDOT
PC71BM device on the other hand shows a higher PCE (2.2%), compared with inverted structure device (1.4%). The JSC of both types of PDPP[Py]2-EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM devices are very similar (5.1 mA cm−2versus 4.9 mA cm−2), while the VOC and FF of the standard structure device are both slightly higher (0.80 V versus 0.70 V and 0.54 versus 0.41 respectively). Despite a relatively high VOC (0.97 V for standard and 0.91 V for inverted structure), PDPP[Ph]2-EDOT
PC71BM devices are very similar (5.1 mA cm−2versus 4.9 mA cm−2), while the VOC and FF of the standard structure device are both slightly higher (0.80 V versus 0.70 V and 0.54 versus 0.41 respectively). Despite a relatively high VOC (0.97 V for standard and 0.91 V for inverted structure), PDPP[Ph]2-EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM devices gave a very moderate PCE of 1.7% for both architecture devices largely due to the low JSC (4.1 mA cm−2 for standard and 4.4 mA cm−2 for inverted structures).
PC71BM devices gave a very moderate PCE of 1.7% for both architecture devices largely due to the low JSC (4.1 mA cm−2 for standard and 4.4 mA cm−2 for inverted structures).
| Device | J SC (mA cm−2) | V OC (V) | FF | PCE (%) |
|---|---|---|---|---|
| Standard PDPP[T]2-EDOT | 6.8 ± 0.3 | 0.25 ± 0.01 | 0.40 ± 0.01 | 0.7 ± 0.1 |
| Standard PDPP[Py]2-EDOT | 5.1 ± 0.4 | 0.80 ± 0.01 | 0.54 ± 0.01 | 2.2 ± 0.2 |
| Standard PDPP[Ph]2-EDOT | 4.1 ± 0.2 | 0.97 ± 0.01 | 0.47 ± 0.01 | 1.7 ± 0.1 |
| Inverted PDPP[T]2-EDOT | 15.5 ± 0.2 | 0.32 ± 0.01 | 0.50 ± 0.01 | 2.5 ± 0.1 |
| Inverted PDPP[Py]2-EDOT | 4.9 ± 0.2 | 0.70 ± 0.01 | 0.41 ± 0.01 | 1.4 ± 0.1 |
| Inverted PDPP[Ph]2-EDOT | 4.4 ± 0.1 | 0.91 ± 0.01 | 0.43 ± 0.01 | 1.7 ± 0.1 |
Fig. 3c and d present the dark J–V characteristics for both standard and inverted architectures. For standard structure devices, the current leakage values of PDPP[Py]2-EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM and PDPP[Ph]2-EDOT
PC71BM and PDPP[Ph]2-EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM devices are about 2 orders of magnitude lower than that of the PDPP[T]2-EDOT
PC71BM devices are about 2 orders of magnitude lower than that of the PDPP[T]2-EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM device, with the high leakage of standard PDPP[T]2-EDOT
PC71BM device, with the high leakage of standard PDPP[T]2-EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM device likely affecting the FF of this device. In comparison, for the inverted structure, the dark current value of the PDPP[T]2-EDOT device is strongly suppressed, and is one order of magnitude lower than that of the PDPP[Py]2-EDOT device.
PC71BM device likely affecting the FF of this device. In comparison, for the inverted structure, the dark current value of the PDPP[T]2-EDOT device is strongly suppressed, and is one order of magnitude lower than that of the PDPP[Py]2-EDOT device.
The external quantum efficiency (EQE) curves of devices are presented in Fig. 3e and f, which agree well with the reported JSC values. The low band gap PDPP[T]2-EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM device based on an inverted structure has a broad photo-response from 350 nm to over 1000 nm with the highest EQE value of 51.6% at 415 nm. Despite the lower band gap of PDPP[T]2-EDOT, the inverted PDPP[T]2-EDOT
PC71BM device based on an inverted structure has a broad photo-response from 350 nm to over 1000 nm with the highest EQE value of 51.6% at 415 nm. Despite the lower band gap of PDPP[T]2-EDOT, the inverted PDPP[T]2-EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM still has higher EQE values than the inverted devices based on the other two polymers. In the standard configuration, the peak EQE is highest for the highest band gap material, with the PDPP[T]2-EDOT
PC71BM still has higher EQE values than the inverted devices based on the other two polymers. In the standard configuration, the peak EQE is highest for the highest band gap material, with the PDPP[T]2-EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM device having the lowest peak EQE. The fact that the peak EQE values are generally less than 50% (and below 30% for the case of (PDPP[Py]2-EDOT and PDPP[Ph]2-EDOT)) indicates that the relatively low efficiencies of these cells compared to current high performance systems are due to relatively low quantum efficiency of charge collection.
PC71BM device having the lowest peak EQE. The fact that the peak EQE values are generally less than 50% (and below 30% for the case of (PDPP[Py]2-EDOT and PDPP[Ph]2-EDOT)) indicates that the relatively low efficiencies of these cells compared to current high performance systems are due to relatively low quantum efficiency of charge collection.
Morphology
In order to understand the relationship between the device performance, morphology, and chemical structure, the microstructure of these PDPP–EDOT copolymer![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) fullerene blends have been probed with a series of characterization techniques. Atomic force microscopy (AFM) was used to image the surface features while transmission electron microscopy (TEM) was employed to image the bulk morphology. Synchrotron-based grazing incidence wide-angle X-ray scattering (GIWAXS) was employed to study thin-film crystallinity with near-edge X-ray absorption fine structure (NEXAFS) spectroscopy also used to assess surface composition.
fullerene blends have been probed with a series of characterization techniques. Atomic force microscopy (AFM) was used to image the surface features while transmission electron microscopy (TEM) was employed to image the bulk morphology. Synchrotron-based grazing incidence wide-angle X-ray scattering (GIWAXS) was employed to study thin-film crystallinity with near-edge X-ray absorption fine structure (NEXAFS) spectroscopy also used to assess surface composition.
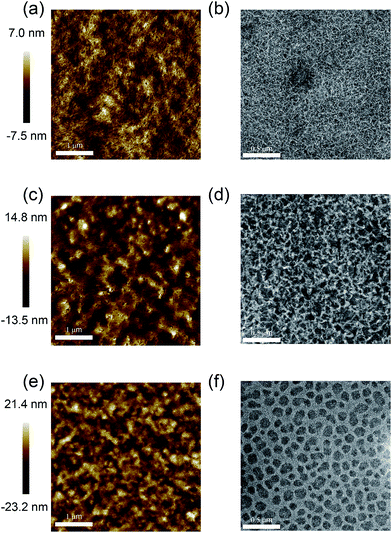
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM film (Fig. 4a) shows a smooth surface and fibrous structures with a surface roughness (Rq, root mean squared) value of 2.0 nm. In comparison, the surface features become larger and rougher in the PDPP[Py]2-EDOT
PC71BM film (Fig. 4a) shows a smooth surface and fibrous structures with a surface roughness (Rq, root mean squared) value of 2.0 nm. In comparison, the surface features become larger and rougher in the PDPP[Py]2-EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM film (Fig. 4b) with a surface roughness of Rq = 4.0 nm. A dramatic change is observed for the PDPP[Ph]2-EDOT
PC71BM film (Fig. 4b) with a surface roughness of Rq = 4.0 nm. A dramatic change is observed for the PDPP[Ph]2-EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM blend, Fig. 4e, which is characterised by relatively large surface undulations with a surface roughness of Rq = 6.3 nm. The AFM images suggest variations in the underlying morphology which are confirmed by the TEM images. For the PDPP[T]2-EDOT
PC71BM blend, Fig. 4e, which is characterised by relatively large surface undulations with a surface roughness of Rq = 6.3 nm. The AFM images suggest variations in the underlying morphology which are confirmed by the TEM images. For the PDPP[T]2-EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM film (Fig. 4b) a fine continuous fibrous structure is seen indicating a relatively fine phase separated structure and consistent with the smooth surface observed with AFM. Measurement of fibril width yielded an average fibril width of 9 ± 3 nm. Some larger features are observed which are attributed to polymer aggregates and are likely associated with the higher regions seen in the AFM image. A coarser phase-separated structure is observed for the PDPP[Py]2-EDOT
PC71BM film (Fig. 4b) a fine continuous fibrous structure is seen indicating a relatively fine phase separated structure and consistent with the smooth surface observed with AFM. Measurement of fibril width yielded an average fibril width of 9 ± 3 nm. Some larger features are observed which are attributed to polymer aggregates and are likely associated with the higher regions seen in the AFM image. A coarser phase-separated structure is observed for the PDPP[Py]2-EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM film (Fig. 4d) with average fibril width of 23 ± 4 nm. A different morphology is observed in TEM image of the PDPP[Ph]2-EDOT
PC71BM film (Fig. 4d) with average fibril width of 23 ± 4 nm. A different morphology is observed in TEM image of the PDPP[Ph]2-EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM film as shown in Fig. 4f, with dark, enclosed domains of average diameter of 70 ± 10 nm surrounded by an interconnected phase with average with of 30 ± 10 nm. Due to the higher density of the fullerene, the enclosed domain with darker contrast is likely to be fullerene-rich with the continuous phase polymer rich. The variations in thin film morphology observed by TEM go some way to explain the observed trends in device performance. Indeed, Li et al. have previously established a relationship between fibril width and solar cell performance in polymer
PC71BM film as shown in Fig. 4f, with dark, enclosed domains of average diameter of 70 ± 10 nm surrounded by an interconnected phase with average with of 30 ± 10 nm. Due to the higher density of the fullerene, the enclosed domain with darker contrast is likely to be fullerene-rich with the continuous phase polymer rich. The variations in thin film morphology observed by TEM go some way to explain the observed trends in device performance. Indeed, Li et al. have previously established a relationship between fibril width and solar cell performance in polymer![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) fullerene blends based on DPP polymers.22 For the polymers investigated here, the PDPP[T]2-EDOT
fullerene blends based on DPP polymers.22 For the polymers investigated here, the PDPP[T]2-EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM blend has the finest morphology with narrowest fibril width, consistent with the high EQEs achieved in inverted PDPP[T]2-EDOT
PC71BM blend has the finest morphology with narrowest fibril width, consistent with the high EQEs achieved in inverted PDPP[T]2-EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM devices. With increased fibril width, the PDPP[Py]2-EDOT
PC71BM devices. With increased fibril width, the PDPP[Py]2-EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM blend has a lower maximum EQE than PDPP[T]2-EDOT-based devices. Interestingly the PDPP[Ph]2-EDOT
PC71BM blend has a lower maximum EQE than PDPP[T]2-EDOT-based devices. Interestingly the PDPP[Ph]2-EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM film does not have a fibrillar morphology, rather having a morphology suggestive of liquid–liquid phase separation seen in other systems such as the MDMO-PPV
PC71BM film does not have a fibrillar morphology, rather having a morphology suggestive of liquid–liquid phase separation seen in other systems such as the MDMO-PPV![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC61BM and PTB7-Th
PC61BM and PTB7-Th![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM where spherical, fullerene-rich domains are surrounded by a continuous polymer-rich phase.23,24 In PDPP
PC71BM where spherical, fullerene-rich domains are surrounded by a continuous polymer-rich phase.23,24 In PDPP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PCBM blends the use of a solvent additive such as DIO is usually effective in suppressing liquid–liquid phase separation22,25 however for the case of the PDPP[Ph]2-EDOT
PCBM blends the use of a solvent additive such as DIO is usually effective in suppressing liquid–liquid phase separation22,25 however for the case of the PDPP[Ph]2-EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM blend this does not seem to be the case. van Franeker25 have recently found that for a PDPP
PC71BM blend this does not seem to be the case. van Franeker25 have recently found that for a PDPP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PCBM blend that the presence of the solvent additive DIO induces aggregation of polymer chains suppressing liquid–liquid separation. This observation may suggest that PDPP[Ph]2-EDOT chains with phenyl flanking units have a reduced tendency to aggregate, which is supported by UV-vis measurements of neat PDPP[Ph]2-EDOT films21 and of PDPP[Ph]2-EDOT
PCBM blend that the presence of the solvent additive DIO induces aggregation of polymer chains suppressing liquid–liquid separation. This observation may suggest that PDPP[Ph]2-EDOT chains with phenyl flanking units have a reduced tendency to aggregate, which is supported by UV-vis measurements of neat PDPP[Ph]2-EDOT films21 and of PDPP[Ph]2-EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM blends that exhibit a lack of vibrational structure. In contrast for the more strongly aggregating PDPP[Py]2-EDOT and PDPP[T]2-EDOT chains fibrillar morphologies are observed resulting from the increased tendency of these chains to aggregate. Thus the choice of flanking unit not only strongly affects the optical properties of these polymers but it also strongly influences the thin-film morphologies achieved in blends with PC71BM. Certainly for the case of the PDPP[Ph]2-EDOT
PC71BM blends that exhibit a lack of vibrational structure. In contrast for the more strongly aggregating PDPP[Py]2-EDOT and PDPP[T]2-EDOT chains fibrillar morphologies are observed resulting from the increased tendency of these chains to aggregate. Thus the choice of flanking unit not only strongly affects the optical properties of these polymers but it also strongly influences the thin-film morphologies achieved in blends with PC71BM. Certainly for the case of the PDPP[Ph]2-EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM the coarse morphology observed is limiting cell efficiency, with morphological feature much greater than the exciton diffusion length. For the PDPP[Py]2-EDOT
PC71BM the coarse morphology observed is limiting cell efficiency, with morphological feature much greater than the exciton diffusion length. For the PDPP[Py]2-EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM blend the large fibril widths observed there (23 ± 4 nm) are also not optimal for cell performance. Therefore it appears that the morphologies achieved so far are limiting the overall efficiency achieved with these polymers, particularly for the PDPP[Py]2-EDOT and PDPP[Ph]2-EDOT-based cells. Interestingly Li et al.22 also correlated fibril width with solubility, with less soluble DPP-based polymers giving narrower fibrils. Higher molecular weight polymers also lead to a reduced solubility which in turn results in a smaller fibre width.26 Out of the three polymers, the DPP derivatives with a six-membered ring as the aryl flanking units, i.e.PDPP[Py]2-EDOT and PDPP[Ph]2-EDOT, show a significantly lower tendency to aggregate (PDPP[T]2-EDOT is also the most crystalline, see below) leading to a worse fibre network formation. Even for the highest performing PDPP[T]2-EDOT
PC71BM blend the large fibril widths observed there (23 ± 4 nm) are also not optimal for cell performance. Therefore it appears that the morphologies achieved so far are limiting the overall efficiency achieved with these polymers, particularly for the PDPP[Py]2-EDOT and PDPP[Ph]2-EDOT-based cells. Interestingly Li et al.22 also correlated fibril width with solubility, with less soluble DPP-based polymers giving narrower fibrils. Higher molecular weight polymers also lead to a reduced solubility which in turn results in a smaller fibre width.26 Out of the three polymers, the DPP derivatives with a six-membered ring as the aryl flanking units, i.e.PDPP[Py]2-EDOT and PDPP[Ph]2-EDOT, show a significantly lower tendency to aggregate (PDPP[T]2-EDOT is also the most crystalline, see below) leading to a worse fibre network formation. Even for the highest performing PDPP[T]2-EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM blend efficiency could be significantly increased by reducing the average fibril width from ∼10 nm to ∼5 nm (ref. 22) such as through the use of higher molecular weight samples. Thus these results highlight the importance of intermolecular interactions in determining nanoscale morphology and hence overall cell performance.
PC71BM blend efficiency could be significantly increased by reducing the average fibril width from ∼10 nm to ∼5 nm (ref. 22) such as through the use of higher molecular weight samples. Thus these results highlight the importance of intermolecular interactions in determining nanoscale morphology and hence overall cell performance.
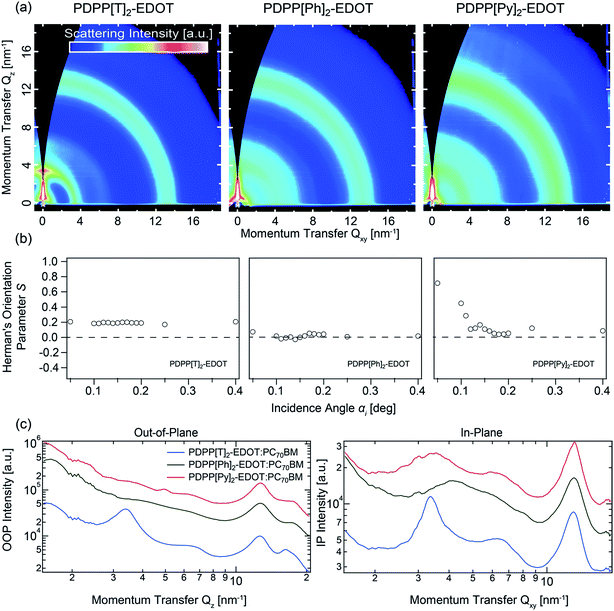
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM blends assessing the thin-film crystallinity. Despite the apparent ordered fibrillar network seen in the TEM images (and similar to previous results22) the GIWAXS patterns indicate a low degree of crystalline ordering in the polymer phase compared to other systems such as regioregular P3HT
PC71BM blends assessing the thin-film crystallinity. Despite the apparent ordered fibrillar network seen in the TEM images (and similar to previous results22) the GIWAXS patterns indicate a low degree of crystalline ordering in the polymer phase compared to other systems such as regioregular P3HT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) fullerene films. The scattering patterns are largely dominated by the scattering of PC71BM aggregates (halo at ∼1.3 Å−1) though polymer scattering features can also be discerned. PDPP[T]2-EDOT appears to be the most highly ordered on the 3 polymers, with a clear alkyl lamellar stacking peak observed at 0.34 Å−1 consistent with previous bulk WAXS measurements on the neat polymer.21 For the other two polymers, an alkyl lamellar stacking peak can only barely be discerned, being much broader and consistent with a significantly shorter crystalline coherence length. The peak positions of the alkyl lamellar stacking peaks for PDPP[Py]2-EDOT and PDPP[Ph]2-EDOT are consistent with the bulk SAXS measurements of ∼0.40 Å−1 and 0.37 Å−1 with the different lamellar stacking distances for the different polymers attributed to the different backbone conformations. In particular the thiophene-flanked PDPP with the highest lamellar stacking distance (∼18.4 Å) has a more coplanar conformation compared to the phenyl-flanked PDPP which has a more twisted backbone conformation and a smaller inter-lamellar distance (∼15.9 Å).
fullerene films. The scattering patterns are largely dominated by the scattering of PC71BM aggregates (halo at ∼1.3 Å−1) though polymer scattering features can also be discerned. PDPP[T]2-EDOT appears to be the most highly ordered on the 3 polymers, with a clear alkyl lamellar stacking peak observed at 0.34 Å−1 consistent with previous bulk WAXS measurements on the neat polymer.21 For the other two polymers, an alkyl lamellar stacking peak can only barely be discerned, being much broader and consistent with a significantly shorter crystalline coherence length. The peak positions of the alkyl lamellar stacking peaks for PDPP[Py]2-EDOT and PDPP[Ph]2-EDOT are consistent with the bulk SAXS measurements of ∼0.40 Å−1 and 0.37 Å−1 with the different lamellar stacking distances for the different polymers attributed to the different backbone conformations. In particular the thiophene-flanked PDPP with the highest lamellar stacking distance (∼18.4 Å) has a more coplanar conformation compared to the phenyl-flanked PDPP which has a more twisted backbone conformation and a smaller inter-lamellar distance (∼15.9 Å).
From the χ-dependence (polar angle-dependence) of the lamellar stacking peak it is possible to calculate Herman's orientational parameter, S, to quantify the orientation of crystallites relative to the substrate.27 The calculated values for S range from S = −0.5 to S = 1 corresponding to fully face-on or fully edge-on, respectively, with S = 0 indicating no preferential orientation. S is plotted as a function of X-ray angle of incidence α based on the analysis of 2D GIWAXS patterns taken at different grazing angles ranging from α = 0.05° to α = 0.40°. For angles below the critical angle, identified as αC = 0.17° in this case based on the angle which gives the highest scattering intensity, scattering is primarily from the top 10 nm of the film, while for angles above the critical angle X-rays scatter from throughout the film. For the PDPP[T]2-EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM blend, a value of S = 0.2 is determined independent of angle of incidence indicating that the PDPP[T]2-EDOT crystallites have a slight edge-on preference that is uniform throughout the depth of the film. For the PDPP[Py]2-EDOT
PC71BM blend, a value of S = 0.2 is determined independent of angle of incidence indicating that the PDPP[T]2-EDOT crystallites have a slight edge-on preference that is uniform throughout the depth of the film. For the PDPP[Py]2-EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM blend a consistent value of S ∼ 0 is calculated for different values of α indicating that the PDPP[Py]2-EDOT crystallites are randomly oriented throughout the film. Finally, for the PDPP[Ph]2-EDOT
PC71BM blend a consistent value of S ∼ 0 is calculated for different values of α indicating that the PDPP[Py]2-EDOT crystallites are randomly oriented throughout the film. Finally, for the PDPP[Ph]2-EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM blend, a strong α-dependence of S is observed, with S ranging from 0.8 at the surface (indicating a strong edge-on orientation preference) to S = 0 within the bulk, indicating no strong orientational preference lower in the film.
PC71BM blend, a strong α-dependence of S is observed, with S ranging from 0.8 at the surface (indicating a strong edge-on orientation preference) to S = 0 within the bulk, indicating no strong orientational preference lower in the film.
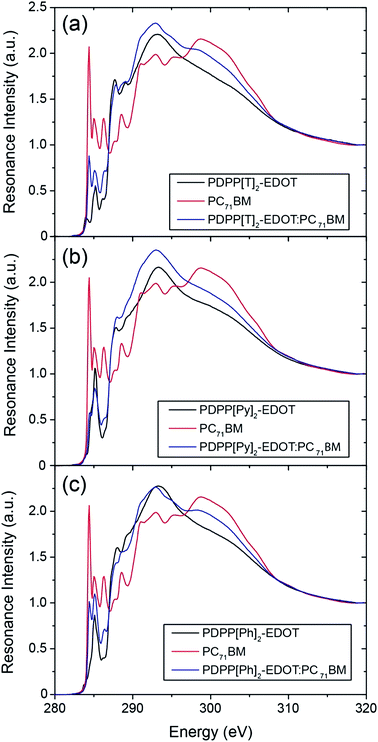
By comparing the blend spectra with that of the reference neat spectra, it is clear that the blend spectra better resemble the neat polymer spectra indicating that the top surface is polymer-rich. By fitting the blend spectra to a linear combination of neat spectra it is possible to determine a %-surface composition. Interestingly, when fitting over the full spectral range the best-fit linear combination deviates from the measured blend spectrum in each case, see ESI.† This deviation is attributed to the polymer chains having a different surface conformation in the neat films compared to blends, with more side-chains exposed to the surface in the blends compared to the neat films, which changes the weighting of the spectra in the π* and σ* regions. To account for this discrepancy, in order to obtain reliable chemical compositions, fits have been made over (i) the entire spectral window (280 eV to 320 eV), (ii) over the π* region (282 eV to 287 eV) and (iii) over the σ* region (287 to 320 eV). Details of these fits can be found in the electronic ESI.† Based on these fits, the surface composition of the PDPP[T]2-EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM blend was determined to be 78 ± 9 wt% polymer, the surface composition of the PDPP[Py]2-EDOT
PC71BM blend was determined to be 78 ± 9 wt% polymer, the surface composition of the PDPP[Py]2-EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM blend to be 92 ± 8 wt% polymer and the surface composition of the PDPP[Ph]2-EDOT
PC71BM blend to be 92 ± 8 wt% polymer and the surface composition of the PDPP[Ph]2-EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM blend to be 70 ± 3 wt% polymer.
PC71BM blend to be 70 ± 3 wt% polymer.
Discussion
For the series of PDPP–EDOT polymers investigated here, the polymer with thiophene flanking units achieved the highest efficiency despite having the lowest VOC. The superior performance of the PDPP[T]2-EDOT-based cells are largely due to the higher JSC with the other polymers achieving higher VOC and FF values. The high JSC of the PDPP[T]2-EDOT-based devices is a product of the higher EQE values obtained and the broader spectral response, with PDPP[T]2-EDOT having an optical gap of 1.13 eV, one of the lowest band gaps reported for a DPP polymer.20,30 While the low bandgap accounts for the broad spectral coverage, the higher EQE values for the PDPP[T]2-EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM cells can be attributed to the more optimal morphology of PDPP[T]2-EDOT
PC71BM cells can be attributed to the more optimal morphology of PDPP[T]2-EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM blends, with a narrower fibril width and superior crystalline structure. Furthermore, space-charge limited current measurements of mobility in a diode configuration give a hole mobility of ∼3 × 10−4 cm2 V−1 s−1 for PDPP[T]2-EDOT compared to ∼2 × 10−6 cm2 V−1 s−1 for PDPP[Py]2-EDOT and ∼4 × 10−5 cm2 V−1 s−1 for PDPP[Ph]2-EDOT.21 This superior hole mobility also allows for PDPP[T]2-EDOT
PC71BM blends, with a narrower fibril width and superior crystalline structure. Furthermore, space-charge limited current measurements of mobility in a diode configuration give a hole mobility of ∼3 × 10−4 cm2 V−1 s−1 for PDPP[T]2-EDOT compared to ∼2 × 10−6 cm2 V−1 s−1 for PDPP[Py]2-EDOT and ∼4 × 10−5 cm2 V−1 s−1 for PDPP[Ph]2-EDOT.21 This superior hole mobility also allows for PDPP[T]2-EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM cells to have a thicker active layer thickness (∼100 nm) compared to the other blends (∼40 to 60 nm) that enables improved light harvesting without a severe increase in recombination. The performance of PDPP[T]2-EDOT-based devices could be further improved either by better control over processing, by pairing PDPP[T]2-EDOT with a more optimum acceptor that would promote a higher VOC, or by improving light absorption particularly in the 600 nm to 800 nm region such as with a retroreflective foil.31 It is also interesting to note that the PDPP[T]2-EDOT
PC71BM cells to have a thicker active layer thickness (∼100 nm) compared to the other blends (∼40 to 60 nm) that enables improved light harvesting without a severe increase in recombination. The performance of PDPP[T]2-EDOT-based devices could be further improved either by better control over processing, by pairing PDPP[T]2-EDOT with a more optimum acceptor that would promote a higher VOC, or by improving light absorption particularly in the 600 nm to 800 nm region such as with a retroreflective foil.31 It is also interesting to note that the PDPP[T]2-EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM cells have a much higher efficiency in the inverted configuration compared to the standard device configuration. The reason for this difference is not clear, since hole and electron mobilities are expected to be relatively balanced for the PDPP[T]2-EDOT and PC71BM, though enrichment of polymer at the top surface may favour an inverted geometry. It is noted that the standard device architecture can lead to greater parasitic absorption particularly in the NIR region compared to inverted devices as observed by Hendriks et al.20 While Hendriks et al. used PEDOT
PC71BM cells have a much higher efficiency in the inverted configuration compared to the standard device configuration. The reason for this difference is not clear, since hole and electron mobilities are expected to be relatively balanced for the PDPP[T]2-EDOT and PC71BM, though enrichment of polymer at the top surface may favour an inverted geometry. It is noted that the standard device architecture can lead to greater parasitic absorption particularly in the NIR region compared to inverted devices as observed by Hendriks et al.20 While Hendriks et al. used PEDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS and LiF/Al layers in their standard devices, the use of a calcium electrode here will produce greater parasitic absorption compared to Al. In particular, Ca only has a maximum reflectivity of 80% over visible to NIR wavelengths32 which will likely result in significant absorption in the top electrode. Silver electrodes in contrast act as a very efficient back reflector at NIR wavelengths. Thus careful choice of electrodes and interlayers and interlayer thicknesses must be considered with the implementation of NIR absorbing polymers.
PSS and LiF/Al layers in their standard devices, the use of a calcium electrode here will produce greater parasitic absorption compared to Al. In particular, Ca only has a maximum reflectivity of 80% over visible to NIR wavelengths32 which will likely result in significant absorption in the top electrode. Silver electrodes in contrast act as a very efficient back reflector at NIR wavelengths. Thus careful choice of electrodes and interlayers and interlayer thicknesses must be considered with the implementation of NIR absorbing polymers.
(C) Experimental section
Materials
The synthesis of PDPP[T]2-EDOT, PDPP[Py]2-EDOT and PDPP[Ph]2-EDOT have been reported previously.21 PC71BM was purchased from Nano-C, while polyethylenimine, 80% ethoxylated solution, 1,8-diiodooctane (98%) and zinc acetate dehydrate (99.999% trace metals basis) were all purchased from Sigma-Aldrich.Device fabrication
The standard device structure employed was ITO/MoOx/active layer/Ca/Al while the inverted device structure was ITO/ZnO/PEIE/active layer/MoOx/Ag. For standard devices MoOx was thermally evaporated in vacuo (∼10−6 mbar) onto cleaned ITO/glass with a thickness of 15 nm. For inverted devices, a 0.073 M ZnO precursor solution was prepare by dissolving 160 mg of zinc acetate dehydrate in 61 mg of ethanolamine and 10 mL of 2-methoxyethanol with vigorous stirring for 12 hours for the hydrolysis reaction at 60 °C. 0.073 M ZnO solution was then spin-coated onto cleaned ITO/glass at 3000 rpm for 30 s and annealed on hot plate at 200 °C for 30 minutes to form a thin conducting layer. Subsequently a 0.4 wt% PEIE solution was spin-coated at 5000 rpm for 30 s and annealed on hot plate at 110 °C for 15 min to form a thin hole-blocking layer. The active layers of the standard devices were all spin-coated from blend solutions at 4000 rpm for 1 min for standard devices, and 4000 rpm, 6000 rpm and 8000 rpm for 1 min for inverted PDPP[T]2-EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM, PDPP[Py]2-EDOT
PC71BM, PDPP[Py]2-EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM and PDPP[Ph]2-EDOT
PC71BM and PDPP[Ph]2-EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM devices respectively. The solvent employed was chloroform with 2 vol% DIO additive and total concentration of the blend solutions were all set at 12 mg mL−1 with a PDPP–EDOT
PC71BM devices respectively. The solvent employed was chloroform with 2 vol% DIO additive and total concentration of the blend solutions were all set at 12 mg mL−1 with a PDPP–EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM ratio of 1
PC71BM ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. The average active layer thickness were 96 nm, 68 nm and 65 nm for standard devices and 96 nm, 61 nm and 40 nm for inverted structure PDPP[T]2-EDOT
2. The average active layer thickness were 96 nm, 68 nm and 65 nm for standard devices and 96 nm, 61 nm and 40 nm for inverted structure PDPP[T]2-EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM, PDPP[Py]2-EDOT
PC71BM, PDPP[Py]2-EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM and PDPP[Ph]2-EDOT
PC71BM and PDPP[Ph]2-EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM devices respectively. A 15 nm Ca layer and a 100 nm Al layer for standard structure devices or a 15 nm MoOx layer and a 100 nm Ag layer for inverted structure devices were subsequently evaporated in vacuo (∼10−6 mbar) through a shadow mask to define electrodes with an active area of 4.5 mm2. All processing steps subsequent to the weighing of solid organic semiconductor powders were conducted in a nitrogen glove box. The devices were encapsulated with epoxy resin and glass cover slides before being removed from the glove box for testing.
PC71BM devices respectively. A 15 nm Ca layer and a 100 nm Al layer for standard structure devices or a 15 nm MoOx layer and a 100 nm Ag layer for inverted structure devices were subsequently evaporated in vacuo (∼10−6 mbar) through a shadow mask to define electrodes with an active area of 4.5 mm2. All processing steps subsequent to the weighing of solid organic semiconductor powders were conducted in a nitrogen glove box. The devices were encapsulated with epoxy resin and glass cover slides before being removed from the glove box for testing.
Device characterization
The photovoltaic characteristics of the solar cells were measured with a Keithley 2635 source measurement unit under a 1 sun, AM1.5G spectrum from a Photo Emission Tech. SS50AA solar simulator. The intensity of simulated solar light was calibrated by using a calibrated silicon solar cell with KG3 filter to obtain an accurate light intensity. External quantum efficiency (EQE) was measured as a function of wavelength by using light from a tungsten halogen lamp dispersed through a monochromator and focused onto the cell. The power density calibration was performed by placing a calibrated photodiode under test position and referencing the intensity measured to that of another silicon photodiode that samples a portion of the beam via a beam-splitter and serves to account for any intensity fluctuations.UV-vis-NIR absorption spectroscopy was measured over the wavelength range from 300 to 1200 nm with a PerkinElmer Lambda 950 Spectrometer. Surface topography was measured with a Veeco Nanoscope V atomic force microscope (AFM) using ScanAsyst mode. TEM images were obtained using a JEOL JEM-2100F TEM operating at a voltage of 200 kV. Defocussed bright-field images were collected using a Gatan UltraScan 1000 (2k × 2k) CCD camera. A 20 μm objective aperture and a large value of defocus (−10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 nm) were employed to increase contrast between the phases.
000 nm) were employed to increase contrast between the phases.
(D) Conclusions
We have studied the photovoltaic performance and morphology of polymer solar cells based on a series of novel EDOT-containing DPP polymers. By varying the aryl flanking units on the DPP core from thiophene, to pyridine to phenyl a range of optical bandgaps spanning 1.91 to 1.13 eV was achieved, attributed to variations in backbone planarity. Despite having the lowest open circuit voltage, the thiophene flanked polymer exhibited the highest efficiency of 2.5% attributed to the more favourable morphology, crystallinity and mobility of PDPP[T]2-EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM blends that enabled EQEs of over 50%. The performance of inverted PDPP[T]2-EDOT
PC71BM blends that enabled EQEs of over 50%. The performance of inverted PDPP[T]2-EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM devices were superior to that of standard devices attributed in part to the superior optical properties of the top electrode used in the inverted structure.
PC71BM devices were superior to that of standard devices attributed in part to the superior optical properties of the top electrode used in the inverted structure.
Acknowledgements
We acknowledge financial support from DFG (SFB 840) and the Bavarian State Ministry of Education, Science and the Arts (Solar technologies go hybrid). C. J. M. thanks the Fonds der Chemischen Industrie for funding the PhD with a Kekule' scholarship and the German National Academic Foundation for other support during the PhD Support from the Elitenetzwerk Bayern (ENB), Macromolecular Science is also kindly acknowledged. Work in Australia was supported by the Australian Research Council (grants FT100100275, DP13012616). This research was undertaken in part on the SAXS/WAXS and Soft X-ray beamlines at the Australian Synchrotron, Victoria, Australia. This work was performed in part at the Melbourne Centre for Nanofabrication (MCN) in the Victorian Node of the Australian National Fabrication Facility (ANFF). The authors acknowledge use of facilities within the Monash Centre for Electron Microscopy.References
- C. W. Tang, US4164431 A, 1979.
- C. W. Tang, Appl. Phys. Lett., 1986, 48, 183 CrossRef CAS.
- D. E. Markov, E. Amsterdam, P. W. M. Blom, A. B. Sieval and J. C. Hummelen, J. Phys. Chem. A, 2005, 109, 5266–5274 CrossRef CAS PubMed.
- P. W. M. Blom, V. D. Mihailetchi, L. J. A. Koster and D. E. Markov, Adv. Mater., 2007, 19, 1551–1566 CrossRef CAS.
- J. J. M. Halls, C. A. Walsh, N. C. Greenham, E. A. Marseglia, R. H. Friend, S. C. Moratti and A. B. Holmes, Nature, 1995, 376, 498–500 CrossRef CAS.
- G. Yu, J. Gao, J. C. Hummelen, F. Wudl and A. J. Heeger, Science, 1995, 270, 1789–1791 CAS.
- G. Yu and A. J. Heeger, J. Appl. Phys., 1995, 78, 4510 CrossRef CAS.
- Y. Liu, J. Zhao, Z. Li, C. Mu, W. Ma, H. Hu, K. Jiang, H. Lin, H. Ade and H. Yan, Nat. Commun., 2014, 5, 5293 CrossRef CAS PubMed.
- J.-D. Chen, C. Cui, Y.-Q. Li, L. Zhou, Q.-D. Ou, C. Li, Y. Li and J.-X. Tang, Adv. Mater., 2015, 27, 1035–1041 CrossRef CAS PubMed.
- S. Zhang, L. Ye, W. Zhao, B. Yang, Q. Wang and J. Hou, Sci. China: Chem., 2015, 58, 248–256 CrossRef CAS.
- M. C. Scharber, D. Mühlbacher, M. Koppe, P. Denk, C. Waldauf, A. J. Heeger and C. J. Brabec, Adv. Mater., 2006, 18, 789–794 CrossRef CAS.
- H. Zhou, L. Yang, A. C. Stuart, S. C. Price, S. Liu and W. You, Angew. Chem., Int. Ed., 2011, 50, 2995–2998 CrossRef CAS PubMed.
- P.-L. T. Boudreault, A. Najari and M. Leclerc, Chem. Mater., 2011, 23, 456–469 CrossRef CAS.
- S. Qu and H. Tian, Chem. Commun., 2012, 48, 3039–3051 RSC.
- W. Li, Y. An, M. M. Wienk and R. A. J. Janssen, J. Mater. Chem. A, 2015, 3, 6756–6760 CAS.
- C. B. Nielsen, M. Turbiez and I. McCulloch, Adv. Mater., 2013, 25, 1859–1880 CrossRef CAS PubMed.
- K. H. Hendriks, G. H. L. Heintges, V. S. Gevaerts, M. M. Wienk and R. A. J. Janssen, Angew. Chem., Int. Ed., 2013, 52, 8341–8344 CrossRef CAS PubMed.
- J.-H. Kim, M. Lee, H. Yang and D.-H. Hwang, J. Mater. Chem. A, 2014, 2, 6348–6352 CAS.
- H. Choi, S.-J. Ko, T. Kim, P.-O. Morin, B. Walker, B. H. Lee, M. Leclerc, J. Y. Kim and A. J. Heeger, Adv. Mater., 2015, 27, 3318–3324 CrossRef CAS PubMed.
- K. H. Hendriks, W. Li, M. M. Wienk and R. A. J. Janssen, J. Am. Chem. Soc., 2014, 136, 12130–12136 CrossRef CAS PubMed.
- C. J. Mueller, C. R. Singh and M. Thelakkat, J. Polym. Sci., Part B: Polym. Phys., 2016, 54, 639–648 CrossRef CAS.
- W. Li, K. H. Hendriks, A. Furlan, W. S. C. Roelofs, M. M. Wienk and R. A. J. Janssen, J. Am. Chem. Soc., 2013, 135, 18942–18948 CrossRef CAS PubMed.
- C. R. McNeill, B. Watts, L. Thomsen, W. J. Belcher, A. K. D. Kilcoyne, N. C. Greenham and P. C. Dastoor, Small, 2006, 2, 1432–1435 CrossRef CAS PubMed.
- B. A. Collins, Z. Li, J. R. Tumbleston, E. Gann, C. R. McNeill and H. Ade, Adv. Energy Mater., 2013, 3, 65–74 CrossRef CAS.
- J. J. van Franeker, M. Turbiez, W. Li, M. M. Wienk and R. A. J. Janssen, Nat. Commun., 2015, 6, 6229 CrossRef CAS PubMed.
- J. J. van Franeker, G. H. L. Heintges, C. Schaefer, G. Portale, W. Li, M. M. Wienk, P. van der Schoot and R. A. J. Janssen, J. Am. Chem. Soc., 2015, 137, 11783–11794 CrossRef CAS PubMed.
- L. A. Perez, P. Zalar, L. Ying, K. Schmidt, M. F. Toney, T.-Q. Nguyen, G. C. Bazan and E. J. Kramer, Macromolecules, 2014, 47, 1403–1410 CrossRef CAS.
- L.-L. Chua, M. Dipankar, S. Sivaramakrishnan, X. Gao, D. Qi, A. T. S. Wee and P. K. H. Ho, Langmuir, 2006, 22, 8587–8594 CrossRef CAS PubMed.
- J. Stöhr, NEXAFS Spectroscopy, Springer, Berlin, 1992 Search PubMed.
- Z. Zeng, Y. Li, J. Deng, Q. Huang and Q. Peng, J. Mater. Chem. A, 2014, 2, 653–662 CAS.
- S. Esiner, T. Bus, M. M. Wienk, K. Hermans and R. A. J. Janssen, Adv. Energy Mater., 2013, 3, 1013–1017 CrossRef CAS.
- A. K. Pandey, P. E. Shaw, I. D. W. Samuel and J.-M. Nunzi, Appl. Phys. Lett., 2009, 94, 103303 CrossRef.
- N. M. Kirby, S. T. Mudie, A. M. Hawley, D. J. Cookson, H. D. T. Mertens, N. Cowieson and V. Samardzic-Boban, J. Appl. Crystallogr., 2013, 46, 1670–1680 CrossRef CAS.
- I. Ilavsky, J. Appl. Crystallogr., 2012, 45, 324–328 CrossRef.
- B. C. C. Cowie, A. Tadich and L. Thomsen, AIP Conf. Proc., 2010, 1234, 307–310 CrossRef.
- B. Watts, L. Thomsen and P. C. Dastoor, J. Electron Spectrosc. Relat. Phenom., 2006, 151, 105–120 CrossRef CAS.
- E. Gann, C. R. McNeill, A. Tadich, B. C. C. Cowie and L. Thomsen, J. Synchrotron Radiat., 2016, 23, 374–380 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ta10078j |
| This journal is © The Royal Society of Chemistry 2016 |