Electrodeposition on pyrite from copper(i) cyanide electrolyte
Abstract
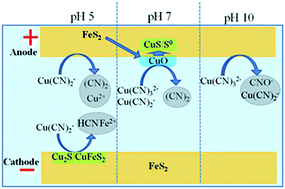
Cyanide and copper often co-exist in process water with complicated speciation. The deposition of copper on pyrite from cyanide bearing solutions is unknown and is the objective of this study. The electrochemical behaviour of pyrite in a solution with CN/Cu mole ratio of 3/1 has been investigated in different solution pH values. It was found that, at pH 7, cuprous cyanide can be oxidized to Cu(II) oxide/hydroxide which deposit on the pyrite surface and transform to Cu(I)-sulfide during the subsequent cathodic sweeping. However, this anodic deposition process is inhibited either at pH 10 due to the dissolution of solid Cu(II)/Cu(I)-species by Cu(CN)32−, or at pH 5 due to the high solubility of Cu(II) oxide/hydroxide. On the other hand, Cu(CN)2− can be deposited onto pyrite, forming a Cu(I)-sulfide layer at more reducing potentials. The understanding of the electrodeposition mechanisms provides opportunities for the recovery of copper from cyanide-bearing waste water, as well as the separation of pyrite from other raw materials by altering its surface properties.

 Please wait while we load your content...
Please wait while we load your content...