Development of an eco-friendly immunochromatographic test strip and its application in detecting Hg2+ without chelators
Suyan Wang†
a,
Limin Wang†*b,
Hongfu Chenb,
Yulong Wangb,
Jia Caib,
Mingming Yangb and
Fengquan Liu*a
aInstitute of Plant Protection, Jiangsu Academy of Agricultural Science, Nanjing, 210014, P. R. China. E-mail: wlm@njau.edu.cn; fqliu20011@sina.com
bCollege of Plant Protection (Key Laboratory of Integrated Management of Crop Diseases and Pests), Nanjing Agricultural University, Nanjing, 210095, P. R. China
First published on 14th January 2016
Abstract
Here, a specific anti-Hg2+ monoclonal antibody (mAb) was generated and an eco-friendly immunochromatographic test strip (EFITS) based on a mAb-nanogold probe for rapid and specific detection of Hg2+ in water was developed. In the method, the conjugation of Hg–MNA (6-mercaptonicotinic acid)–BSA (bovine serum albumin) was synthesized as an immunogen, the conjugation of MNA–OVA (ovalbumin) was synthesized and selected as a coating antigen. The specific anti-Hg2+ mAb from BALB/C female mice was screened based on a competitive immunoassay. The coating antigen and goat anti-mouse IgG antibody were coated on a nitrocellulose membrane (NC membrane) as a test line and a control line, respectively. The anti-Hg2+ mAb-nanogold probe was applied to the conjugate pad. Hg2+ competes with OVA–MNA for the mAb-nanogold probe causing a color change on the test line corresponding to the Hg2+ content. Thus, we can distinguish the subtle differences through a strip reader. The resulting EFITS is able to detect Hg2+ with LOD of 0.4 ng mL−1 at 9 min by quantitative analysis. EFITS demonstrated here is eco-friendly (without Hg2+ on the strip), capable of rapid detection, and does not require chelators.
1. Introduction
With the rapid development of industrialization, the mercury ion, which is highly toxic and can have adverse effects on human health, has been frequently detected in the environment.1,2 Research has shown that dietary exposure to mercury in drinking water or sea food can seriously affect the function of the immune system, cardiovascular system, kidneys, lungs, bones and nervous tissues in mammals.3–6 Minamata disease has alerted us the importance of monitoring mercury levels in our surroundings. Therefore, sensitive and rapid analytical methods for detecting Hg2+ levels in water samples are crucial for monitoring water quality.7So far, traditional methods such as atomic fluorescence spectroscopy,8 atomic absorption spectroscopy,9,10 inductively coupled plasma spectroscopy-mass spectrometry,11,12 High Performance Liquid Chromatography (HPLC) and the HPLC-ICP-MS13,14 coupling technique have been sensitive and accurate. However, these methods called for a high dependence on laboratory techniques requiring expensive and sophisticated instruments together with technical experts. All these restrict their extended application in the routine detection of heavy metals. In this context, lots of effort has been made to develop novel, cheap, simple, portable and real time detection methods to detect mercury.
Immunochromatographic test strips had attracted much attention because of their rapid, low-cost and convenient character.15–18 With the integration of colloidal gold and antigen–antibody reaction, we can detect the target analytes through a visible color reaction. Thus, the immunochromatographic test strips played a potential role in point of care assay to monitor our environment and human health.
To date, the developed immunochromatographic test strips for detecting heavy metals needed chelating agent to bind heavy metals and usually the strips contain heavy metals.2,19–22 In this research, a monoclonal antibody (mAb) was produced by using the Hg–MNA–BSA as immunogen, which can specifically recognize individual Hg2+ without any chelating agent. Based on the mAb, a high specific and sensitive immunochromatograpic strip was developed to detect the individual Hg2+ from water. More importantly, the OVA–MNA (ovalbumin–6-mercaptonicotinic acid) was used as coating antigen, which without any heavy metal, resulting in realizing an eco-friendly and chelator-free strip for quantitative detection of Hg2+ in water samples. The developed eco-friendly immunochromatographic test strip enabled a rapid and quantitative detection of Hg2+ in 10 min. The novelty of the developed EFITS is eco-friendly (without Hg2+ on strip), chelator-free and fast detection.
2. Materials and methods
2.1. Reagents and equipments
Gold nanoparticles (40 nm) were produced in the plant quarantine and applied immunology laboratory of Nanjing Agricultural University (Nanjing, China). 6-Mercaptonicotinic acid (MNA), bovine serum albumin (BSA), ovalbumin (OVA), Tween-20, dimethyl sulfoxide (DMSO), 3,3′,5,5′-tetramethylbenzidine (TMB), dimethyl formamide (DMF), N-hydroxysuccinimide (NHS), N,N′-dicycohexylcarbodimide (DCC), Freund's complete/incomplete adjuvants and polyethylene glycol (PEG1500) were purchased from Sigma chemical Co. (St. Louis, USA). Ammonium carbonate, calcium chloride, manganese sulfate, lead sulfate, zinc sulfate, magnesium sulfate, ferric chloride, methylmercury chloride (CH3HgCl), mercury(I) chloride (Hg2Cl2) and mercuric sulfate (HgSO4) were purchased from Aladdin industrial corporation. Hypoxanthine aminopterin thymidine (HAT), hypoxanthine thymidine (HT) and culture media Dulbecco's Modified Eagle Medium (DMEM) were provided by Gibco (USA). Horseradish peroxidase labeled goat anti-mouse IgG conjugate (HRP-GaMIgG) was bought from Boster Biological Technology Co., Ltd (Wuhan, China). Fetal bovine serum (FBS) was provided by Hangzhou “Sijiqing” company (Hangzhou, China). Ultra-pure deionized water was produced with a triple-distilled water system, and used to prepare all aqueous solutions. NC membranes, glass fibers and absorbent pads were purchased from Millipore Corp (Billerica, MA, USA). Bicinchoninic acid kit was purchased from sigma.An XYZ3060 dispensing platform and CM4000 Guillotine Cutter (BioDot, Irvine, CA) were used to prepare test strips. Samples were validated using an Agilent 1260 HPLC system (Agilent Technologies, Santa Clara, CA). A membrane strip reader (TSR5000) was purchased from Jiening Biotech Co. Ltd (Shanghai, China). SP2/0 cells were stored in the plant quarantine and applied immunology laboratory of Nanjing Agricultural University (Nanjing, China), and BALB/c mice were purchased from the Center of Comparative Medicine of Yangzhou University (Yangzhou, China). All animals used in this study and animal experiments were approved by the Department of Science and Technology of Jiangsu Province. The license number was SYXK (SU) 2010-0005.
2.2. Synthesis of MNA–protein conjugation
The MNA was conjugated to BSA or OVA by the DCC/NHS ester method according to the previous literature with a slight modification.23,24 Briefly, MNA (0.014 g), NHS (0.011 g), and DCC (0.071 g) were dissolved in DMF (900 μL) and the reaction was stirred overnight at room temperature. After centrifugation of the solution at 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 rpm for 15 min, the supernatant was dropwise added to 7 mL of 0.13 mol L−1 NaHCO3 solution containing 117 mg BSA or OVA and kept stirring for 4 h at room temperature. Then, the resulting solution was centrifuged and the supernatant was dialyzed in 0.01 mol L−1 PBS at 4 °C for 2 days with five-times change of buffer. The protein concentrations of the MNA–protein (BSA or OVA) conjugations were determined by BCA kit.
400 rpm for 15 min, the supernatant was dropwise added to 7 mL of 0.13 mol L−1 NaHCO3 solution containing 117 mg BSA or OVA and kept stirring for 4 h at room temperature. Then, the resulting solution was centrifuged and the supernatant was dialyzed in 0.01 mol L−1 PBS at 4 °C for 2 days with five-times change of buffer. The protein concentrations of the MNA–protein (BSA or OVA) conjugations were determined by BCA kit.
The MNA–OVA solution was used as one of a potential coating antigen, and both the MNA–BSA and MNA–OVA were used to preparation of Hg–MNA–protein conjugation.
2.3. Preparation of Hg–MNA–protein conjugation
CH3HgCl (0.07 mmol) was dissolved in 540 μL of methanol containing 10% of 1 mol L−1 NaOH (v/v). The solution of CH3HgCl was added dropwise to MNA–protein (BSA or OVA) while stirring and the reaction was incubated overnight at room temperature. The solution was dialyzed in 0.01 M (NH4)2CO3 for 2 days with five-times change of buffer. The protein concentrations of the Hg–MNA–protein (BSA or OVA) conjugations were determined by a Nanodrop 1000 UV-VIA. The Hg–MNA–BSA was used as immunogen, Hg–MNA–OVA was used as the second potential coating antigen.2.4. Production of monoclonal antibody
Five BALB/C female mice of about 7 weeks old were immunized with the Hg–MNA–BSA conjugation. The first dose consisted of 100 μg of conjugation intraperitoneally injected as an emulsion of PBS and Freund's complete adjuvant. The subsequent injections were emulsified in Freund's incomplete adjuvant. The second booster immunization was given to each mouse at 3 week intervals after the initial immunization, the third immunization was given at 4 week intervals and the following immunization was given at 8 week intervals. One week after the last injection, the antisera were obtained from the tail vein of each mouse. The sera were tested for antibody titers and for analyte (Hg2+) recognition by indirect incompetitive/competitive ELISA. The process of ELISA was performed as described previously.24The mouse showing the highest serum immuno-reactivity was given a peritoneal cavity injection of 100 μg Hg–MNA–BSA in PBS at 1 week intervals. Three to four days after the last injection, the donor mouse was sacrificed. SP2/0 murine myeloma cells were cultured in DMEM supplemented with 20% FBS. Splenocytes of selected mice were harvested aseptically. Cell fusion and hybridoma selection procedures were performed essentially as described previously.25 The fusion cells were incubated at 37 °C with 5% CO2, and after 7 days, the supernatants were screened by an indirect ELISA using Hg–MNA–OVA and OVA–MNA as coating antigen. The supernatants which can recognize both coating antigens were detected for further indirect competitive ELISA using Hg2+ as competitor. The hybridomas whose supernatants recognized the two coating antigens and could be inhibited by Hg2+ were subcloned for three times using the limiting dilution method.25 Four stable antibody-producing clones were expanded and cryopreserved in liquid nitrogen. Abundant antibodies were collected and subjected to purification by ammonium sulfate precipitation. The unpurified mAb was stored at −20 °C in the presence of 50% glycerol. The purified mAb was stored at −20 °C.
2.5. Preparation of nanogold-mAb probe
The nanogold-mAb probe was prepared according to the method26 with a little change. In brief, the pH value of the colloidal solution was adjusted to 8.2 with 0.2 M K2CO3. 66 μL of 2.5 mg mL−1 solution were added into 10 mL adjusted colloidal solution with quick stir for 5 min and kept for 1 h at room temperature. The amount of the mAb was 10% more than the optimal amount which was determined referring to the method.27 Afterwards, 1% BSA (final concentration) was added to stabilize the mixture with quick stir for 5 min. After one-hour standing at room temperature, the solution was centrifuged (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm) at 4 °C for 25 min, and the supernatant was carefully sucked up. The bottom sediment was resuspended with 2 mL 2% BSA containing 0.01 M sodium borate and centrifuged (10
000 rpm) at 4 °C for 25 min, and the supernatant was carefully sucked up. The bottom sediment was resuspended with 2 mL 2% BSA containing 0.01 M sodium borate and centrifuged (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm) at 4 °C for 25 min to remove the redundant regent. The washing procedure was repeated twice, followed by a last washing using a solution containing 2% BSA, 3% sucrose, 0.01 M sodium borate and 0.05% sodium azide. Finally, the sediment was resuspended with 1 mL of the last washing solution and the prepared gold-mAb solution was stored at 4 °C for future study in one month.
000 rpm) at 4 °C for 25 min to remove the redundant regent. The washing procedure was repeated twice, followed by a last washing using a solution containing 2% BSA, 3% sucrose, 0.01 M sodium borate and 0.05% sodium azide. Finally, the sediment was resuspended with 1 mL of the last washing solution and the prepared gold-mAb solution was stored at 4 °C for future study in one month.
2.6. Preparation of the immunochromatographic test strip
As shown in Fig. 1, the immunochromatographic test strip is made up of four parts including a sample pad, a conjugation pad, a nitrocellulose membrane, and an absorbent pad.26,28 The nanogold-mAb probe was dispensed by XYZ 3060 onto the dried, 2% BSA, 0.01 M sodium borate blocked glass fiber pad and vacuumized at 37 °C for 30 min. The potential coating antigens were diluted with 0.09% NaCl and dispensed at test (T) line, while 0.15 mg mL−1 goat anti-mouse IgG was dispensed at control (C) line when the NC membrane was dried at 37 °C for 2 h. The distance between the T and C line was about 5 mm. The NC membrane, conjugate pad, sample pad and absorbent pad were pasted onto the PVC plate correctly which was then cut into 4 mm-wide strips using the programmable strip cutter CM4000. All strips were sealed in a plastic bag with a pack of desiccant gel and stored at 4 °C.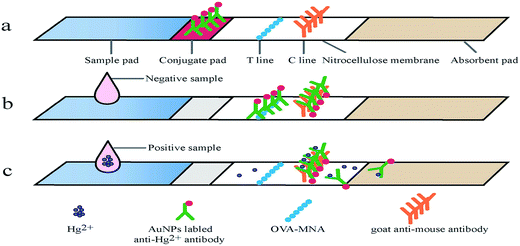 | ||
| Fig. 1 The schematic diagram of an eco-friendly immunochromatographic test strip (EFITS) and a competitive immunoreaction on the EFITS. | ||
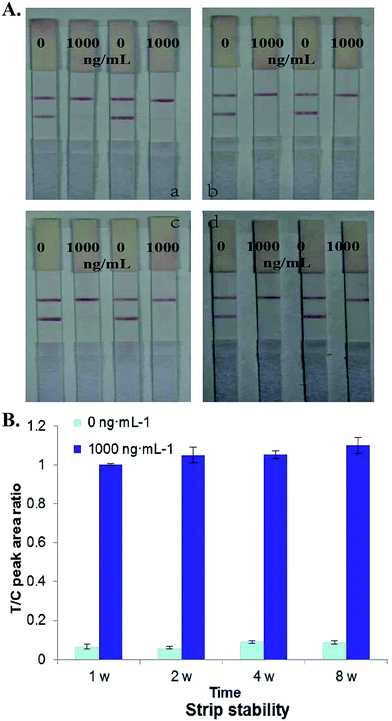
2.7. Evaluation of developed immunochromatographic test strip
3. Results and discussion
3.1. Principle of the immunochromatographic test strip
The principle of the immunochromatographic test strip was based on competitive immunoreaction (Fig. 1). The nanogold-mAb probe, which can bind to the coating antigen on the test line, was used as the labeling material. During the lateral chromatography, the coating antigen (immobilized on test line) acted as a competitive analogue of Hg2+, binding to the nanogold-mAb. When a sample solution is applied to the sample pad, the liquid sample can flow to the other end of the strip according to the capillary action. The nanogold-mAb immobilized on conjugation zone flow together with sample fluid to reach the test line and control line. For positive experiment, signal intensity of the test line (red line from nanogold) showed an inverse proportional corresponding relationship with Hg2+content. The excess analyte and probe contained in fluid fraction and continued flowing onto the control line and the absorbent pad. The probe interacts with goat anti-mouse IgG immobilized on control line to form a red line. For negative experiment, the probe can be fully binded to the coating antigen on test line and goat anti-mouse IgG on control line with the same color intensity. The ratio of color intensity of the test line and control line (T/C) was quantified at 9 min by using a test strip reader.3.2. The affinity analysis of mAb to MNA–OVA and individual Hg2+
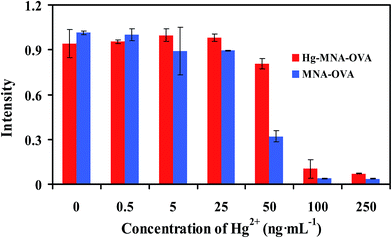
2 potential coating antigens, MNA–OVA and Hg–MNA–OVA, were synthesized for development of the immunochromatographic test strip. The affinity analysis of mAb to coating antigens and individual Hg2+ was performed on the immunochromatographic test strip (Fig. 2). As illustrated in Fig. 2, when the concentration of individual Hg2+ was 0 ng mL−1, the intensity demonstrated the affinity of nanogold-mAb to MNA–OVA and Hg–MNA–OVA. With the increase of Hg2+, the intensity decreased because individual Hg2+ is inhibiting the binding site of nanogold-mAb so that the intensity of test line is decreasing.From Fig. 2, without Hg2+, there was no significant difference in the affinity of nanogold-mAb toward MNA–OVA and Hg–MNA–OVA. With the increasing of Hg2+, individual Hg2+ showed stronger competition towards nanogold-mAb when competing with MNA–OVA than that with Hg–MNA–OVA. That means mAb has stronger affinity toward Hg–MNA–OVA than MNA–OVA, in the meantime, it can be competed by individual Hg2+ without any chelator. More importantly, the coating antigen MNA–OVA (in absence of Hg2+) can be used as coating antigen on the immunochromatographic test strip, so the developed test strip for detection of Hg2+ was called eco-friendly immunochromatographic test strip (EFITS).
3.3. Optimization of analytical parameters for the EFITS
Ionic strength (0–1.0 M), Tween-20 (0.05–0.4%) and pH values (5.5–9.0) were optimized to obtain the best sensitivity. The results were judged by naked eye. Ionic strength (0 M, 0.05 M, 0.15 M, 0.5 M and 1.0 M NaCl additionally added into 0.01 M PBST, pH 7.4) was optimized to obtain the best sensitivity. As shown in Table 1, with the ionic strength in working solution increased, the sensitivity of the strip improved. However, along with the ionic strength reached 0.15 M, the sensitivity diminished as the ionic strength increased. So, ionic strength of 0.15 M was chosen for future optimization. Extreme pH values may induce antibody structure changes thus destroying paratope of antibody29 and obstructing the binding of antibody with antigen. In this work, as we can see from Table 2, the sensitivity of the one-step strip improved with the pH value of working solution increased. But it reached the highest point at pH value 7.0 and 8.0. However color of T line was a little lighter in pH 8.0 than that of 7.0 while they maintained the same sensitivity. Consulting convenience of detecting mercury, we chose pH 7.0 for further study. As surfactant, moderate Tween-20 can block active group on NC membrane and nonspecific sites of our coating antigen, lowering nonspecific adsorption on NC membrane and enhancing color of T line. We found from Table 3 that with the increase of Tween-20 content, T line signal decreased. That is to say proper increase of Tween-20 content would improve sensitivity. Yet, too much Tween-20 weakened sensitivity. Thus, 0.2% Tween-20 was selected for working solution.| Ionic strength of working solution | Mercury(II) standard concentration (ng mL−1) | |||||
|---|---|---|---|---|---|---|
| 0 | 50 | 250 | 500 | 1000 | ||
| a 7: red line appeared; 6: red line appeared but was weaker than 7; 5: red line appeared but was weaker than 6; 4: red line appeared but was weaker than 5; 3: red line appeared but was weaker than 4; 2: red line appeared but was weaker than 3; 1: red line did not appear. | ||||||
| 0 M NaCI | Test line | 7 | 6 | 5 | 4 | 2 |
| Control line | 7 | 7 | 7 | 7 | 7 | |
| 0.05 M NaCI | Test line | 7 | 6 | 5 | 4 | 2 |
| Control line | 7 | 7 | 7 | 7 | 7 | |
| 0.15 M NaCI | Test line | 7 | 5 | 3 | 2 | 1 |
| Control line | 7 | 7 | 7 | 7 | 7 | |
| 0.5 M NaCI | Test line | 7 | 5 | 4 | 3 | 1 |
| Control line | 7 | 7 | 7 | 7 | 7 | |
| 1.0 M NaCI | Test line | 7 | 6 | 5 | 3 | 1 |
| Control line | 7 | 7 | 7 | 7 | 7 | |
| The pH values of working solution | Mercury(II) standard concentration (ng mL−1) | |||||
|---|---|---|---|---|---|---|
| 0 | 50 | 250 | 500 | 1000 | ||
| a 7: red line appeared; 6: red line appeared but was weaker than 7; 5: red line appeared but was weaker than 6; 4: red line appeared but was weaker than 5; 3: red line appeared but was weaker than 4; 2: red line appeared but was weaker than 2; 1: red line did not appear. | ||||||
| pH 5.5 | Test line | 7 | 6 | 5 | 4 | 2 |
| Control line | 7 | 7 | 7 | 7 | 7 | |
| pH 6.0 | Test line | 7 | 6 | 5 | 4 | 2 |
| Control line | 7 | 7 | 7 | 7 | 7 | |
| pH 7.0 | Test line | 7 | 5 | 3 | 2 | 1 |
| Control line | 7 | 7 | 7 | 7 | 7 | |
| pH 8.0 | Test line | 5 | 5 | 3 | 2 | 1 |
| Control line | 5 | 5 | 5 | 5 | 5 | |
| pH 9.0 | Test line | 5 | 5 | 4 | 2 | 1 |
| Control line | 5 | 5 | 5 | 5 | 5 | |
| Tween-20 (v/v) of working solution | Mercury(II) standard concentration (ng mL−1) | |||||
|---|---|---|---|---|---|---|
| 0 | 50 | 250 | 500 | 1000 | ||
| a 7: red line appeared; 6: red line appeared but was weaker than 7; 5: red line appeared but was weaker than 6; 4: red line appeared but was weaker than 5; 3: red line appeared but was weaker than 4; 2: red line appeared but was weaker than 3; 1: red line did not appear. | ||||||
| 0.05% | Test line | 7 | 6 | 5 | 4 | 2 |
| Control line | 7 | 7 | 7 | 7 | 7 | |
| 0.1% | Test line | 7 | 6 | 5 | 3 | 2 |
| Control line | 7 | 7 | 7 | 7 | 7 | |
| 0.2% | Test line | 7 | 5 | 3 | 2 | 1 |
| Control line | 7 | 7 | 7 | 7 | 7 | |
| 0.4% | Test line | 5 | 5 | 4 | 3 | 1 |
| Control line | 5 | 5 | 5 | 5 | 5 | |
To sum up, the working solution of the developed method contained 0.15 M ionic strength and 0.2% Tween-20 in 0.01 M PBS with pH 7.0.
3.4. The sensitivity of the developed EFITS
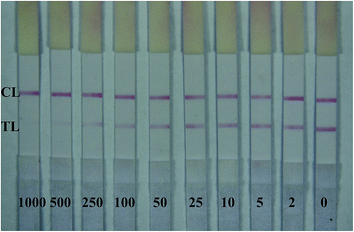
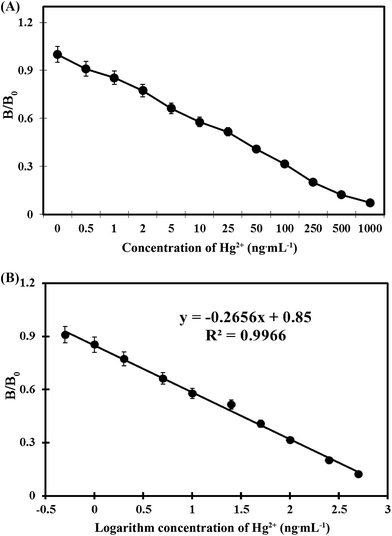
For this one-step strip assay, a series of mercury concentrations were dissolved in optimized working solution. Each solution was added on sample pad (100 μL per sample) and waited for 9 min. Water samples containing mercury standard concentrations ranging from 0 to 1000 ng mL−1 were assayed by our developed strips. As shown in Fig. 3, with mercury concentration increased, the intensity of red color on T line reduced. With mercury concentration of 5 ng mL−1, the intensity of red color on T line was greatly weaker than that at zero concentration.For quantification of Hg2+ on the test strip, mercury standard concentrations ranging from 0 ng mL−1 to 1000 ng mL−1 dissolved in optimized working solution were assayed by strips and the results were scanned by a strip reader. The obtained detection curve is shown in Fig. 4A. From Fig. 4A, we can observe that in the range of 0.5–500 ng mL−1, the diagram between B/B0 and logarithm of mercury concentration (ng mL−1) was linear (Fig. 4B). The regression equation was obtained (y = −0.2656x + 0.85, R2 = 0.9966). The IC50 value was calculated as 20.8 ng mL−1, and the detection limit was 0.4 ng mL−1.
In this study, the detection limit was 0.4 ng mL−1, which is lower than 2 ng mL−1 recommended by United States Environmental Protection agency21 and 6 ng mL−1 recommended by World Health Organization.30 This research provides a new view for detecting heavy metals with monoclonal antibodies. On one hand, we can detect mercury without using any chelators attributing to good quality of mAb. It could be owning to MNA, which contains a cyclic pyridine ring and a sulfhydryl group. MNA bare the mercury ion outside which makes it more likely for antibodies to recognize mercury ions. There is a possibility that unmask heavy metals from carrier proteins would increase probability of generating antibodies toward specific heavy metals. On the other hand, our developed one-step strip can be totally heavy metal-free and won't generate heavy metal pollution to our surrounding. Still, we don't know the principle of how the mAb works without using chelators. But it gives us confidence of the possibility of detecting single heavy metals without using chelators.
3.5. Evaluation of the developed EFITS
| Samples | Theoretical (ng mL−1) | Measure (ng mL−1) | Mean recovery (%) ± SD |
|---|---|---|---|
| Mineral water | 400 | 400.0 | 100.0 ± 10.3 |
| 200 | 204.7 | 102.4 ± 10.3 | |
| 100 | 104.2 | 104.2 ± 8.6 | |
| Tap water | 400 | 407.8 | 102.0 ± 7.2 |
| 200 | 202.1 | 101.0 ± 4.6 | |
| 100 | 105.8 | 105.8 ± 3.5 | |
| Lake water | 200 | 203.6 | 101.8 ± 4.29 |
| 100 | 81.9 | 82.0 ± 3.0 | |
| 50 | 44.3 | 88.7 ± 1.7 | |
| River water | 200 | 191.5 | 95.8 ± 4.8 |
| 100 | 96.2 | 96.2 ± 7.2 | |
| 50 | 46.5 | 93.1 ± 3.6 |
| Metal ions and related compounds | IC50 (ng mL−1) | CR (%) |
|---|---|---|
| Hg2+ | 20.8 | 100 |
| Hg+ | >1000 | <0.1 |
| CH3Hg+ | >1000 | <0.1 |
| MNA–Hg | 77 | 27 |
| MNA | 112 | 18.6 |
| Ca2+ | >1000 | <0.1 |
| Cd2+ | >1000 | <0.1 |
| Zn2+ | >1000 | <0.1 |
| Mg2+ | >1000 | <0.1 |
| Pd2+ | >1000 | <0.1 |
| Fe2+ | >1000 | <0.1 |
| Mn2+ | >1000 | <0.1 |
4. Conclusion
In this work, an eco-friendly immunochromatographic test strip (EFITS) and a chelator-free method to detect mercury ion were developed. The quantitative detection limit was 0.4 ng mL−1 measured by a test strip reader. The developed method showed a good specificity and accuracy in application of detecting water samples containing mercury ion. Like most immunochromatographic test strips, the developed one-step strip enabled the detection with 9 min, and the quantified results could be obtained through a membrane strip reader.Acknowledgements
The authors thank Dr Pedro Laborda and Dr Louis Conway for their comments. This work was supported by the National Natural Science Foundation of China (31401771), the National High Technology Research and Development Program of China (2012AA101401-1, 2013AA065601) and the Natural Science Foundation of Jiangsu Province (BK20140685).References
- P. Miretzky and A. F. Cirelli, J. Hazard. Mater., 2009, 167, 10–23 CrossRef CAS PubMed.
- Y. Zhou, Y. S. Li, X. Y. Meng, Y. Y. Zhang, L. Yang, J. H. Zhang, X. R. Wang, S. Y. Lu, H. L. Ren and Z. S. Liu, Sens. Actuators, B, 2013, 183, 303–309 CrossRef CAS.
- Z. Guo, G. Q. Chen, G. M. Zeng, Z. W. Li, A. W. Chen, M. Yan, L. Z. Liu and D. Y. Huang, RSC Adv., 2014, 4, 59275–59283 RSC.
- Y. Liao, Q. Li, N. Wang and S. J. Shao, Sens. Actuators, B, 2015, 215, 592–597 CrossRef CAS.
- X. J. Zhan, T. Xi and P. Zhou, Environ. Forensics, 2013, 14, 103–108 CrossRef CAS.
- M. Y. Zhu, Y. Wang, Y. Deng, L. Yao, S. B. Adeloju, D. D. Pan, F. Xue, Y. C. Wu, L. Zheng and W. Chen, Biosens. Bioelectron., 2014, 61, 14–20 CrossRef CAS PubMed.
- Y. H. Luo, L. L. Xu, A. H. Liang, A. P. Deng and Z. L. Jiang, RSC Adv., 2014, 4, 19234–19237 RSC.
- K. Leopold, M. Foulkes and P. J. Worsfold, Anal. Chem., 2009, 81, 3421–3428 CrossRef CAS PubMed.
- C. Burrini and A. Cagnini, Talanta, 1997, 44, 1219–1223 CrossRef CAS PubMed.
- L. P. Yu, J. Agric. Food Chem., 2005, 53, 9656–9662 CrossRef CAS PubMed.
- B. M. W. Fong, T. S. Siu, J. S. K. Lee and S. Tam, J. Anal. Toxicol., 2007, 31, 281–287 CrossRef CAS PubMed.
- J. L. Gómez-Ariza, F. Lorenzo and T. García-Barrera, Anal. Bioanal. Chem., 2005, 382, 485–492 CrossRef PubMed.
- A. Dago, O. Gonzalez-Garcia, C. Arino, J. M. Diaz-Cruz and M. Esteban, J. Chromatogr. A, 2009, 1216, 6752–6757 CrossRef CAS PubMed.
- J. L. Gomez-Ariza, D. Sanchez-Rodas, I. Giraldez and E. Morales, Analyst, 2000, 125, 401–407 RSC.
- Y. S. Li, Y. Zhou, S. Y. Lu, D. J. Guo, H. L. Ren, X. M. Meng, B. H. Zhi, C. Lin, Z. Wang, X. B. Li and Z. S. Liu, Food Control, 2012, 24, 72–77 CrossRef CAS.
- S. H. Huang, Sens. Actuators, B, 2007, 127, 335–340 CrossRef CAS.
- Q. K. Fang, L. M. Wang, Q. Cheng, J. Cai, Y. L. Wang, M. M. Yang, X. D. Hua and F. Q. Liu, Anal. Chim. Acta, 2015, 881, 82–89 CrossRef CAS PubMed.
- C. R. Xing, L. Q. Liu, S. S. Song, M. Feng, H. Kuang and C. L. Xu, Biosens. Bioelectron., 2015, 66, 445–453 CrossRef CAS PubMed.
- K. Abe, K. Nakamura, T. Arao, Y. Sakurai, A. Nakano, C. Suginuma, K. Tawarada and K. Sasaki, J. Sci. Food Agric., 2011, 91, 1392–1397 CrossRef CAS PubMed.
- A. M. Lopez-Marzo, J. Pons and D. A. Blake, Biosens. Bioelectron., 2013, 47, 190–198 CrossRef CAS PubMed.
- Y. Zhou, X. L. Tian, Y. S. Li, Y. Y. Zhang, L. Yang, J. H. Zhang, X. R. Wang, S. Y. Lu, H. L. Ren and Z. S. Liu, Biosens. Bioelectron., 2011, 30, 310–314 CrossRef CAS PubMed.
- Y. Zhou, Y. Y. Zhang, F. G. Pan, Y. S. Li, S. Y. Lu, H. L. Ren, Q. F. Shen, Z. H. Li, J. H. Zhang, Q. J. Chen and Z. S. Liu, Biosens. Bioelectron., 2010, 25, 2534–2538 CrossRef CAS PubMed.
- F. D. Cai, Q. Zhu, K. Zhao, A. P. Deng and J. G. Li, Environ. Sci. Technol., 2015, 49, 5013–5020 CrossRef CAS PubMed.
- Y. Z. Wang, H. Yang, M. Pschenitza, R. Niessner, Y. Li, D. Knopp and A. P. Deng, Anal. Bioanal. Chem., 2012, 43, 2519–2528 Search PubMed.
- J. C. Howard, G. W. Butcher, G. Galfre and C. Milstein, Monoclonal Anti-Rat MHC (H-1) Alloantibodies, Springer, Berlin Heidelberg, 1979, pp. 54–60 Search PubMed.
- L. M. Wang, J. Cai, Y. L. Wang, Q. K. Fang, S. Y. Wang, Q. Cheng, D. Du, Y. H. Lin and F. Q. Liu, Microchim. Acta, 2014, 181, 1565–1572 CrossRef CAS.
- X. D. Hua, G. L. Qian, J. F. Yang, B. S. Hu, J. Q. Fan, N. Qin, G. Li, Y. Y. Wang and F. Q. Liu, Biosens. Bioelectron., 2010, 26, 189–194 CrossRef CAS PubMed.
- X. Liu, J. J. Xiang, Y. Tang, X. L. Zhang, Q. Q. Fu, J. H. Zou and Y. H. Lin, Anal. Chim. Acta, 2012, 745, 99–105 CrossRef CAS PubMed.
- R. Reverberi and L. Reverberi, J. Blood Transfus., 2007, 5(4), 227 Search PubMed.
- H. He, F. Wu, M. J. Xu, S. G. Yang, C. Sun and Y. H. Yang, Anal. Methods, 2011, 3, 1859–1864 RSC.
Footnote |
| † The first two authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2016 |