Ru-Catalyzed selective C–H oxidative olefination with N-heteroarenes directed by pivaloyl amide†
Li
Zhang
,
Changpeng
Chen
,
Jian
Han
,
Zhi-Bin
Huang
* and
Yingsheng
Zhao
*
Key Laboratory of Organic Synthesis of Jiangsu Province, College of Chemistry, Chemical Engineering and Materials Science, Soochow University, Suzhou 215123, China. E-mail: zbhuang@suda.edu.cn; yszhao@suda.edu.cn
First published on 8th August 2016
Abstract
A highly efficient and practical Ru-catalyzed direct C–H functionalization of indolines and N-alkylaniline with a simple pivaloyl as a directing group is developed. Broad substrate scopes with respect to N-heteroarenes and olefins are observed. The selective C7-position alkynylation for indoline also proceeds well by using inexpensive ruthenium as a catalyst, and pivaloyl as a directing group.
Introduction
The nitrogen-containing scaffolds are especially important as they are the omnipresent component in various natural compounds and biologically active products, pharmaceutical compounds and organic dyes.1 Among these kinds of compounds, the indoline-based alkaloids and aniline derivatives are ubiquitous structural motifs which can be found in various active compounds.2 Thus, highly regioselective direct functionalization of these compounds would be highly important and is in great demand. Therefore, a great number of approaches have been developed to functionalize these compounds in the last few decades.3 For example, the selective oxidative coupling of NH-acetanilides with olefins via Pd- and Rh-catalysts has been well explored.4,5 The group of Carretero reported a general method for the ortho-olefination of N-alkyl aniline derivatives with the 2-pyridylsulfonyl group as the directing group with palladium as a catalyst.6 Recently, the selective C–H functionalization of the C-7 position of indoline derivatives has been disclosed by employing a transition-metal-catalyst.7 Various coupling partners were successfully employed in these directing group assisted C–H functionalizations, such as alkenylation,8 arylation,9 alkynylation,10 allylation,11 amination,12 alkylation13etc. Even though these protocols have emerged, the development of the selective functionalization of the C7-position of indolines or N-alkyl aniline derivatives with less expensive oxidants and cheaper catalysts is still in great demand. Herein we report an oxidative coupling of pivaloyl amide protected indolines and N-alkyl aniline derivatives with olefins via the ruthenium catalyst under mild conditions. Various olefins including acrylates, sulfones, acrylonitriles, phosphonates, vinyl ketones, and allyl alcohols could all tolerate this, affording the corresponding products in moderate to excellent yields.The highly regioselective C–H transformation with the ruthenium complex as a catalyst was first discovered by Oi and Inoue.14 Since then, various research groups, such as the groups of Miura,15 Ackermann,16 Wang,17 Dixneuf,18 Jeganmohan,19 Ramana,20 and Lam,21 have done intensive studies on the ruthenium catalyzed C–H functionalization reactions. Thus, a great number of C–H transformations with various substrates, such as carboxylic acid,22 heterocycle,23 amide,24 alcohol,25etc., were successfully achieved with the ruthenium catalyst. Inspired by these ruthenium-catalyzed C–H transformations, we speculated that the selective olefination of the C7-position of indolines might be realizable by using the inexpensive and stable ruthenium as the catalyst with well explored pivaloyl amide as the directing group. The ortho-olefination of the pivaloyl group protected N-alkylaniline, of which there are few reports of realizing the ortho-C–H functionalization might also be compatible.
Results and discussion
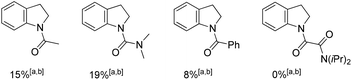
With these conditions in mind, we first treated pivaloyl amide protected indoline 1a with ethyl acrylate 2a in the presence of [RuCl2(p-cymene)]2 (5 mol%), AgSbF6 (20 mol%), and Cu(OAc)2·H2O (1 equiv.) in toluene at 100 °C for 20 h under Ar, the C7-position olefinated product 3a was observed in 7% yield (Table 1, entry 2). We next explored the solvent effect in this oxidative olefination reaction. The solvents including dioxane, acetonitrile, dichloroethane, dichlorideethane, and tert-amyl alcohol (t-AmOH), gave the product 3a in poor results (entries 1–7). Delightfully, tetrahydrofuran showed a great promoting effect in the transformation, affording the desired product 3a in 80% yield (entry 9). We next explored various oxidants to replace the equivalent oxidant copper acetate. However, none of the oxidants could give better results for 3a. When we decreased the reaction temperature to 80 °C, only 63% yield of 3a was achieved (entry 8). Further scanning of the other additives suggested that AgSbF6 was the best catalyst activating agent. The confirming reaction revealed that the ruthenium catalyst is irreplaceable in this transformation. Notably, alternative directing groups or protecting groups, such as Ac, N-benzoyl, dimethylcarbamyl amide and oxalyl amide were also tested under optimized conditions. However, none could give the corresponding products in more than 20% yield.| Entry | Additive | Solvent | T | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: 1a (0.2 mmol), 2a (0.4 mmol), [RuCl2(p-cymene)]2 (5 mol%), Cu(OAc)2·H2O (1 equiv.), additive (20 mol%), solvent (1 mL), 100 °C, 20 h, Ar. b GC yield of 3a was determined using tridecane as an internal standard. c Isolated yield. d Cu(OAc)2·H2O (0.2 equiv.). e O2 as the oxidant. f No catalyst. | ||||
| 1 | AgSbF6 | Dioxane | 100 °C | 18 |
| 2 | AgSbF6 | Toluene | 100 °C | 7 |
| 3 | AgSbF6 | DME | 100 °C | 27 |
| 4 | AgSbF6 | t-AmOH | 100 °C | 20 |
| 5 | AgSbF6 | CH3CN | 100 °C | 12 |
| 6 | AgSbF6 | H2O | 100 °C | 16 |
| 7 | AgSbF6 | DCE | 100 °C | 32 |
| 8 | AgSbF6 | THF | 80 °C | 63 |
| 9 | AgSbF6 | THF | 100 °C | 80 (78)c |
| 10 | AgSbF6 | THF | 100 °C | 21d (<5)e |
| 11 | AgBF4 | THF | 100 °C | 7 |
| 12 | None | THF | 100 °C | <5 |
| 13f | AgSbF6 | THF | 100 °C | 0 |
|
||||
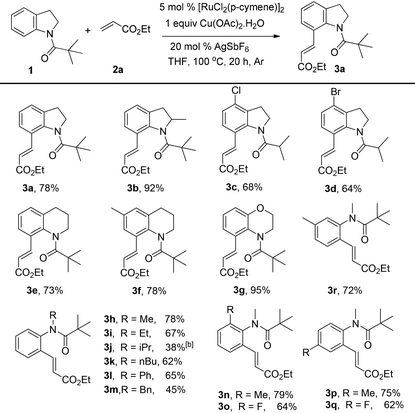
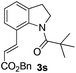
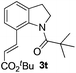
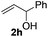
Next, we started to explore the substrate scope of N-heteroarenes under the optimized simple reaction conditions (Table 2). The pivaloyl-protected 2-methylindoline gave the olefinated product 3b in excellent yield. However, a stronger electron-withdrawing substituent afforded lower yield under the standard reaction conditions (3c, 3d). The six-membered N-heteroarenes including 1,2,3,4-tetrahydroquinoline and benzomorpholine were also tested under the optimized reaction conditions, affording the corresponding products in good yields (3e, 3f, 3g). Encouraged by the observed results, we next expanded the substrates to N-alkylaniline, which has few approaches to realize the ortho-C–H functionalization. The simple pivaloyl amide exhibited excellent coordination ability with the ruthenium catalyst to perform the oxidative C–H/C–H olefination. Generally, the steric effect greatly affected the transformation. For example, the less hindered functional groups of methyl, ethyl, and n-butyl gave the corresponding olefinated products in good yields respectively (3h, 3i, 3k). The bulk function groups of isopropyl and benzyl afforded the corresponding products in less than 50% yield (3m, 3j), along with the recovery of the starting material. The ortho, meta, and para substituted N-methylaniline all transformed into olefinated products in good yield.
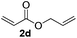
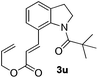
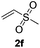
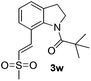
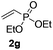
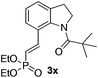
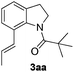
Subsequently, the scope of olefins was examined and the results are listed in Table 3. Generally, the acrylates gave the corresponding olefinated products in good yields (3s, 3t, 3u). Interestingly, both electron rich and deficient olefins could undergo oxidative olefination, selectively affording the products. The results indicated that the electron-deficient olefins are more active than the electron-rich olefins. Further scanning revealed that the other electron-deficient olefins including sulfones, acrylonitriles, phosphonates, and vinyl ketones all proceed well, affording the corresponding products in good to excellent yields (3w, 3x, 3z). Impressively, the allyl alcohol 2h successfully participated in the oxidative olefination reaction, providing 3y in 68% yield. It was probable that the oxidant copper acetate could oxidize the alcohol to ketone during the reaction. Interestingly, allyl acetate could be used as a coupling partner, which gave a quaint olefinated product in good yield (3aa).
| Entry | 2 | 3 products | Yield (%) |
|---|---|---|---|
| a Reaction conditions: 1a (0.2 mmol), 2 (0.4 mmol), [RuCl2(p-cymene)]2 (5 mol%), Cu(OAc)2·H2O (1 equiv.), AgSbF6 (20 mol%), THF (1 mL), 100 °C, 20 h, Ar. | |||
| 1 |
|
|
72 |
| 2 |
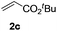
|
|
74 |
| 3 |
|
|
73 |
| 4 |
|
|
75 |
| 5 |
|
|
89 |
| 6 |
|
|
91 |
| 7 |
|
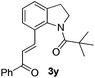
|
68 |
| 8 |
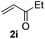
|
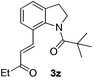
|
81 |
| 9 |
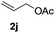
|
|
78 |
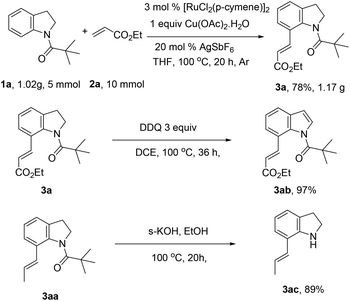
To further demonstrate the synthetic utility of this developed method, gram scale reactions were performed with less amount of catalyst, and an excellent yield of 3a was achieved. As expected, the product could be transformed into indole derivatives in quantitative yield. Finally the pivaloyl directing group could be easily removed under basic conditions, providing the C7-position olefinated product 3ac in 89% yield (Scheme 1).
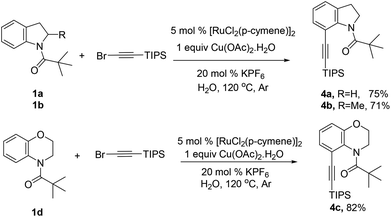
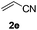
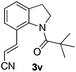
Alkynes are attractive building blocks in synthetic chemistry due to their versatile transformation in the synthesis of multiple functional groups. To the best of our knowledge, there is still no report of the selective alkynylation of the C7 position of indolines with the ruthenium catalyst. To our great delight, the alkynylation could be realized in good yields by using H2O as the solvent and KPF6 as the additive, which meets the requirement of green chemistry. Unfortunately, the alkynylation was not compatible with pivaloyl protected N-alkylaniline substrates (Scheme 2).
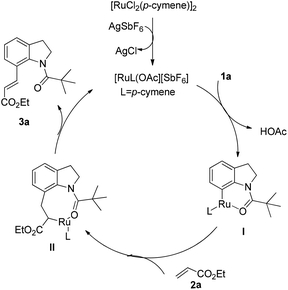
Based on previous reports,26 a plausible mechanism for this oxidative olefination is shown in Scheme 3. First, the [RuCl2(p-cymene)]2 catalyst is activated by Ag+ in the reaction. The ruthenium species coordinated with the pivaloyl directing group, followed by ortho metalation, affording a six-membered metallacycle intermediate I. Regioselective coordinative insertion of olefin into the ruthenium complex provides the intermediate II. Subsequent reductive elimination and β-hydride elimination, generates the olefinated product. The ruthenium complex could be further oxidized to active the ruthenium species with copper acetate as the oxidant.
Conclusions
In summary, we have developed a practical and highly efficient Ru-catalyzed selective C–H functionalization of indolines and N-alkylaniline with a simple pivaloyl as a directing group. Various substrate scopes with respect to N-heteroarenes and olefins are achieved. The C7-position alkynylation is also realized with ruthenium as a catalyst. Further studies on the mechanism are currently underway in our lab.Acknowledgements
This research was supported financially by the Natural Science Foundation of China (no. 21572149) and the Young National Natural Science Foundation of China (no. 21403148). The support of PAPD is also greatly acknowledged.Notes and references
- (a) J. P. Burke, Z. Bian, S. Shaw, B. Zhao, C. M. Goodwin, J. Belmar, C. F. Browning, D. Vigil, A. Friberg, D. V. Camper, O. W. Rossanese, T. Lee, E. T. Olejniczak and S. W. Fesik, J. Med. Chem., 2015, 58, 3794 CrossRef CAS PubMed; (b) Y. Ozawa, K. Kusano, T. Owa, A. Yokoi, M. Asada and K. Yoshimatsu, Cancer Chemother. Pharmacol., 2012, 69, 1353 CrossRef CAS PubMed; (c) A. J. Kochanowska-Karamyan and M. T. Hamann, Chem. Rev., 2010, 110, 4489 CrossRef CAS PubMed; (d) B. M. Trost and M. K. Brennan, Synthesis, 2009, 3003 CrossRef CAS; (e) P. Dydio, T. Zielinski and J. Jurczak, Chem. Commun., 2009, 30, 4560 RSC; (f) Y. Ferandin, K. Bettayeb, M. Kritsanida, O. Lozach, P. Polychronopoulos, P. Magiatis, A.-L. Skaltsounis and L. Meijer, J. Med. Chem., 2006, 49, 4638 CrossRef CAS PubMed; (g) R. Hili and A. K. Yudin, Nat. Chem. Biol., 2006, 2, 284 CrossRef CAS PubMed; (h) R. Mohan, M. Banerjee, A. Ray, T. Manna, L. Wilson, T. Owa, B. Bhattacharyya and D. Panda, Biochemistry, 2006, 45, 5440 CrossRef CAS PubMed; (i) F.-E. Chen and J. Huang, Chem. Rev., 2005, 105, 4671 CrossRef CAS PubMed; (j) D. A. Horton, G. T. Bourne and M. L. Smythe, Chem. Rev., 2003, 103, 893 CrossRef CAS PubMed; (k) D. L. Boger, C. B. Boyce, R. M. Garbaccio and J. A. Goldberg, Chem. Rev., 1997, 97, 787 CrossRef CAS PubMed.
- (a) C. Ma, X. Yang, H. Kandemir, M. Mielczarek, E. B. Johnston, R. Griffith, N. Kumar and P. J. Lewis, ACS Chem. Biol., 2013, 8, 1972 CrossRef CAS PubMed; (b) B. M. Trost and M. K. Brennan, Synthesis, 2009, 3003 CrossRef CAS; (c) Z. Wang and R. Vince, Bioorg. Med. Chem., 2008, 16, 3587 CrossRef CAS PubMed; (d) M. G. Bell, D. L. Gernert, T. A. Grese, M. D. Belvo, P. S. Borromeo, S. A. Kelley, J. H. Kennedy, S. P. Kolis, P. A. Lander, R. Richey, V. S. Sharp, G. A. Stephenson, J. D. Williams, H. Yu, K. M. Zimmerman, M. I. Steinberg and P. K. Jadhav, J. Med. Chem., 2007, 50, 6443 CrossRef CAS PubMed; (e) C. B. Hartline, E. A. Harden, S. L. Williams- Aziz, N. L. Kushner, R. J. Brideau and E. R. Kern, Antiviral Res., 2005, 65, 97 CrossRef CAS PubMed; (f) B. Meseguer, D. Alonso-Díaz, N. Griebenow, T. Herget and H. Waldmann, Angew. Chem., Int. Ed., 1999, 38, 2902 CrossRef CAS; (g) D. R. Beukes, M. T. Davie- Coleman, M. Kelly-Borges, M. K. Harper and D. J. Faulkner, J. Nat. Prod., 1998, 61, 699 CrossRef CAS PubMed; (h) D. L. Boger, C. W. Boyce, R. M. Garbaccio and J. A. Goldberg, Chem. Rev., 1997, 97, 787 CrossRef CAS PubMed; (i) K. Mahmood, M. Pais, C. Fontaine, H. M. Ali, A. H. A. Hadi and E. Gulttet, Tetrahedron Lett., 1993, 34, 1795 CrossRef CAS.
- (a) S. Pan, T. Wakaki, N. Ryu and T. Shibata, Chem. – Asian J., 2014, 9, 1257 CrossRef CAS PubMed; (b) L.-Y. Jiao and M. Oes-treich, Chem. – Eur. J., 2013, 19, 10845 CrossRef CAS PubMed; (c) L.-Y. Jiao and M. Oestreich, Org. Lett., 2013, 15, 5374 CrossRef CAS PubMed; (d) Z. Song, R. Samanta and A. P. Antonchick, Org. Lett., 2013, 15, 5662 CrossRef CAS PubMed; (e) S. De, S. Ghosh, S. Bhunia, J. A. Sheikh and A. Bisai, Org. Lett., 2012, 14, 4466 CrossRef CAS PubMed.
- (a) P. Kannaboina, K. A. Kumar and P. Das, Org. Lett., 2016, 5, 900 CrossRef PubMed; (b) D. Yang, S. Mao, Y.-R. Gao, D.-D. Guo, S.-H. Guo, B. Li and Y.-Q. Wang, RSC Adv., 2015, 5, 23727 RSC; (c) L.-Y. Jiao and M. Oestreich, Org. Lett., 2013, 15, 5374 CrossRef CAS PubMed; (d) L.-Y. Jiao and M. Oestreich, Chem. – Eur. J., 2013, 19, 10845 CrossRef CAS PubMed; (e) T. Nishikata and B. H. Lishutz, Org. Lett., 2010, 12, 1972 CrossRef CAS PubMed; (f) W. Rauf, A. L. Thompson and J. M. Brown, Chem. Commun., 2009, 26, 3874 RSC.
- (a) Z. Song, R. Samanta and A. P. Antonchick, Org. Lett., 2013, 15, 5662 CrossRef CAS PubMed; (b) F. W. Patureau and F. Glorius, J. Am. Chem. Soc., 2010, 132, 9982 CrossRef CAS PubMed; (c) J. Willwacher, S. Rakshit and F. Glorius, Org. Biomol. Chem., 2011, 9, 4736 RSC.
- A. García-Rubia, B. Urones, R. G. Arrayás and J. C. Carretero, Angew. Chem., Int. Ed., 2011, 50, 10921 CrossRef PubMed.
- (a) W. Hou, Y. Yang, W. Ai, Y. Wu, X. Wang, B. Zhou and Y. Li, Eur. J. Org. Chem., 2015, 395 CrossRef CAS; (b) C. Pan, A. Abdukader, J. Han, Y. Cheng and C. Zhu, Chem. – Eur. J., 2014, 20, 3606 CrossRef CAS PubMed; (c) T. Nishikata, A. R. Abela, S. Huang and B. H. Lip-shutz, J. Am. Chem. Soc., 2010, 132, 4978 CrossRef CAS PubMed; (d) Z. Shi, B. Li, X. Wan, J. Cheng, Z. Fang, B. Cao, C. Qin and Y. Wang, Angew. Chem., Int. Ed., 2007, 46, 5554 CrossRef CAS PubMed; (e) D. Kalyani, N. R. Deprez, L. V. Desai and M. S. Sanford, J. Am. Chem. Soc., 2005, 127, 7330 CrossRef CAS PubMed.
- (a) X.-F. Yang, X.-H. Hu, C. Feng and T.-P. Loh, Chem. Commun., 2015, 51, 2532 RSC; (b) D. Yang, S. Mao, Y.-R. Gao, D.-D. Guo, S.-H. Guo, B. Li and Y.-Q. Wang, RSC Adv., 2015, 5, 23727 RSC; (c) S. Pan, T. Wakaki, N. Ryu and T. Shibata, Chem. – Asian J., 2014, 9, 1257 CrossRef CAS PubMed; (d) B. Urones, R. G. Arrayás and J. C. Carretero, Org. Lett., 2013, 15, 1120 CrossRef CAS PubMed; (e) L. Jiao and M. Oestreich, Org. Lett., 2013, 15, 5374 CrossRef CAS PubMed; (f) Z. Song, R. Samanta and A. P. Antonchick, Org. Lett., 2013, 15, 5662 CrossRef CAS PubMed.
- (a) L.-Y. Jiao, P. Smirnov and M. Oestreich, Org. Lett., 2014, 16, 6020 CrossRef CAS PubMed; (b) L. Jiao and M. Oestreich, Chem. – Eur. J., 2013, 19, 10845 CrossRef CAS PubMed; (c) T. Nishikata, A. R. Abela, S. Huang and B. H. Lipshutz, J. Am. Chem. Soc., 2010, 132, 4978 CrossRef CAS PubMed; (d) D. Kalyani, N. R. Deprez, L. V. Desai and M. S. Sanford, J. Am. Chem. Soc., 2005, 127, 7330 CrossRef CAS PubMed; (e) Z. Shi, B. Li, X. Wan, J. Cheng, Z. Fang, B. Cao, C. Qin and Y. Wang, Angew. Chem., Int. Ed., 2007, 46, 5554 CrossRef CAS PubMed.
- (a) Y.-X. Wu, Y.-X. Yang, B. Zhou and Y.-C. Li, J. Org. Chem., 2015, 80, 1946 CrossRef CAS PubMed; (b) X.-F. Yang, X.-H. Hu, C. Feng and T.-P. Loh, Chem. Commun., 2015, 51, 2532 RSC.
- (a) S. Sharma, Y. Shin, N. K. Mishra, J. Park, S. Han, T. Jeong, Y. Oh, Y. Lee, M. Choi and I. S. Kim, Tetrahedron, 2015, 71, 2435 CrossRef CAS; (b) J. Park, N. K. Mishra, S. Sharma, S. Han, Y. Shin, T. Jeong, J. S. Oh, J. H. Kwak, Y. H. Jung and I. S. Kim, J. Org. Chem., 2015, 80, 1818 CrossRef CAS PubMed; (c) M. Kim, S. Sharma, N. K. Mishra, S. Han, J. Park, M. Kim, Y. Shin, J. H. Kwak, S. H. Han and I. S. Kim, Chem. Commun., 2014, 50, 11303 RSC.
- (a) K. Shinand and S. Chang, J. Org. Chem., 2014, 79, 12197 CrossRef PubMed; (b) C. Pan, A. Abdukader, J. Han, Y. Cheng and C. Zhu, Chem. – Eur. J., 2014, 20, 3606 CrossRef CAS PubMed; (c) W. Hou, Y. Yang, W. Ai, Y. Wu, X. Wang, B. Zhou and Y. Li, Eur. J. Org. Chem., 2015, 395 CrossRef CAS; (d) J. Kim, J. Kim and S. Chang, Chem. – Eur. J., 2013, 19, 7328 CrossRef CAS PubMed; (e) M. R. Yadav, R. K. Rit and A. K. Sahoo, Org. Lett., 2013, 15, 1638 CrossRef CAS PubMed.
- (a) S. Pan, S. Ryu and T. Shibata, Adv. Synth. Catal., 2014, 356, 929 CrossRef CAS; (b) W. Ai, X.-Y. Yang, Y.-X. Wu, X. Wang, Y.-C. Li, Y.-X. Yang and B. Zhou, Chem. – Eur. J., 2014, 20, 17653 CrossRef CAS PubMed; (c) S. R. Neufeldt, C. K. Seigerman and M. Sanford, Org. Lett., 2013, 15, 2302 CrossRef CAS PubMed.
- S. Oi, Y. Tanaka and Y. Inoue, Organometallics, 2006, 25, 4773 CrossRef CAS.
- (a) Y. Hashimoto, T. Ortloff, K. Hirano, T. Satoh, C. Bolm and M. Miura, Chem. Lett., 2012, 41, 151 CrossRef CAS; (b) T. Ueyama, S. Mochida, T. Fukutani, K. Hirano, T. Satoh and M. Miura, Org. Lett., 2011, 13, 706 CrossRef CAS PubMed.
- (a) J. Li, C. Kornhaaß and L. Ackermann, Chem. Commun., 2012, 48, 11343 RSC; (b) L. Ackermann, L. Wang, R. Wolfram and A. V. Lygin, Org. Lett., 2012, 14, 728 CrossRef CAS PubMed; (c) K. Graczyk, W. Ma and L. Ackermann, Org. Lett., 2012, 14, 4110 CrossRef CAS PubMed; (d) L. Ackermann, L. H. Wang, R. Wolfram and A. V. Lygin, Org. Lett., 2011, 14, 728 CrossRef PubMed; (e) L. Ackermann and J. Pospech, Org. Lett., 2011, 13, 4153 CrossRef CAS PubMed.
- (a) B. Li, J. Ma, Y. Liang, N. Wang, S. Xu, H. Song and B. Wang, Eur. J. Org. Chem., 2013, 1950 CrossRef CAS; (b) B. Li, J. F. Ma, H. B. Song, S. S. Xu and B. Q. Wang, J. Org. Chem., 2013, 78, 9345 CrossRef CAS PubMed.
- (a) K. S. Singh and P. H. Dixneuf, Organometallics, 2012, 31, 7320 CrossRef CAS; (b) P. B. Arockiam, C. Fischmeister, C. Bruneau and P. H. Dixneuf, Green Chem., 2011, 13, 3075 RSC.
- (a) K. Padala and M. Jeganmohan, Org. Lett., 2012, 14, 1134 CrossRef CAS PubMed; (b) K. Padala and M. Jeganmohan, Org. Lett., 2011, 13, 6144 CrossRef CAS PubMed.
- Y. Goriya and C. V. Ramana, Chem. – Eur. J., 2012, 18, 3288 CrossRef PubMed.
- S. R. Chidipudi, M. D. Wieczysty, I. Khan and H. W. Lam, Org. Lett., 2013, 15, 570 CrossRef PubMed.
- (a) L. Ackermann and J. Pospech, Org. Lett., 2011, 13, 4153 CrossRef CAS PubMed; (b) K. Padala, S. Pimparkar, P. Madasamy and M. Jeganmohan, Chem. Commun., 2012, 48, 7140 RSC; (c) R. K. Chinnagolla and M. Jeganmohan, Chem. Commun., 2012, 48, 2030 RSC.
- (a) C. Tirler and L. Ackermann, Tetrahedron, 2015, 71, 4543 CrossRef CAS; (b) N. Hofmann and L. Ackermann, J. Am. Chem. Soc., 2013, 135, 5877 CrossRef CAS PubMed; (c) J. Li, S. Warratz, D. Zell, S. D. Sarkar, E. E. Ishikawa and L. Ackermann, J. Am. Chem. Soc., 2015, 137, 13894 CrossRef CAS PubMed; (d) L. Ackermann, R. Vicente, H. K. Potukuchi and V. Pirovano, Org. Lett., 2010, 12, 5032 CrossRef CAS PubMed.
- (a) C. S. Kuai, L. H. Wang, H. Y. Cui, J. H. Shen, Y. D. Feng and X. L. Cui, ACS Catal., 2016, 6, 186 CrossRef CAS; (b) J. C. Wu;, W. B. Xu, Z.-X. Yu and J. Wang, J. Am. Chem. Soc., 2015, 137, 9489 CrossRef PubMed.
- (a) L. Ackermann, J. Pospech and H. K. Potukuchi, Org. Lett., 2012, 14, 2146 CrossRef CAS PubMed; (b) V. S. Thirunavukkarasu, M. Donati and L. Ackermann, Org. Lett., 2012, 14, 3417 Search PubMed.
- (a) H. J. Li, X. Y. Xie and L. Wang, Chem. Commun., 2014, 50, 4218 RSC; (b) Q.-Z. Zheng, Y.-F. Liang, C. Qin and N. Jiao, Chem. Commun., 2013, 49, 5654 RSC; (c) K. Padala and M. Jeganmohan, Org. Lett., 2011, 13, 6144 CrossRef CAS PubMed; (d) C. D. Pan, A. Abdukader, J. Han, Y. X. Cheng and C. J. Zhu, Chem. – Eur. J., 2014, 20, 3606 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6qo00327c |
| This journal is © the Partner Organisations 2016 |