Open Access Article
Open Access ArticleCarbo-cyclohexadienes vs. carbo-benzenes: structure and conjugative properties†‡
Arnaud
Rives
ab,
Iaroslav
Baglai
abc,
Cécile
Barthes
ab,
Valérie
Maraval
*ab,
Nathalie
Saffon-Merceron
d,
Alix
Saquet
ab,
Zoia
Voitenko
c,
Yulian
Volovenko
c and
Remi
Chauvin
*ab
aCNRS, LCC (Laboratoire de Chimie de Coordination), 205 route de Narbonne, BP 44099, F-31077 Toulouse Cedex 4, France. E-mail: vmaraval@lcc-toulouse.fr; chauvin@lcc-toulouse.fr; Fax: +33 5 61 55 30 03
bUniversité de Toulouse, UPS, Institut de Chimie de Toulouse, ICT-FR2599, 118 Route de Narbonne, F-31062 Toulouse, France
cKiev National Taras Shevchenko University, 60 Volodymlyrska St, 01033 Kiev, Ukraine
dUniversité de Toulouse, UPS, Institut de Chimie de Toulouse, ICT-FR 2599, 118 route de Narbonne, 31062 Toulouse, France
First published on 7th November 2014
Abstract
Ideally Cs-/C2v-symmetric chromophores, constituted by two electro-active groups conjugated through the carbo-mer of the cyclohexa-1,3-diene core, are selectively prepared by the SnCl2-mediated reduction of tailored hexaoxy-[6]pericyclynes: in the latter substrates, one of the 1,4-dioxybut-2-yne edges is “chemically locked” by two CF3 substituents preventing complete reduction to the corresponding aromatic carbo-benzenic core, which is expected to be more “π-insulating” between the electro-active ends. The bis-trifluoromethylated carbo-cyclohexadiene products are also shown to be significantly stabilized with respect to their bis-phenylated analogues. Their structural (crystal X-ray diffraction analyses), spectroscopical (NMR and UV-vis spectra), physio-optical (dichromism in solution) and electrochemical (cyclic voltammograms) properties are compared on the basis of the electron-donating/electron-withdrawing nature of the substituents. These properties are also compared with those of their aromatic carbo-benzene and flexible carbo-n-butadiene counterparts.
Introduction
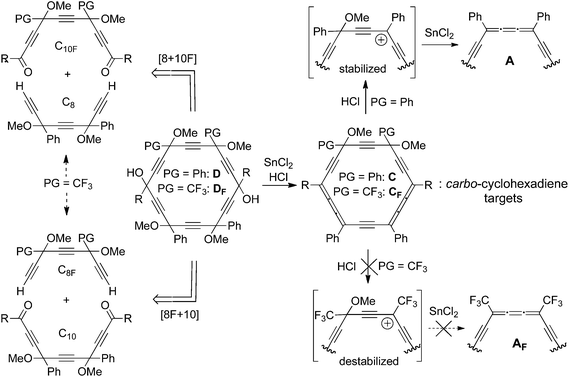
Until the recent past,1 the chemistry of carbo-mers mainly focused on carbo-benzenes,2 because of the stability, rigidity and π-electronic features anticipated to be associated with their unique aromatic structure,3 as compared to those of acyclic fragments. Like the C6 ring of benzene, the C18 macrocycle of carbo-benzenes A (Fig. 1) can be formally divided into two acyclic components relevant from both the viewpoints of experimental retro-synthesis4 and theoretical aromaticity analysis.5 While the ethylene and 1,3-butadiene components for benzene are stable molecules, the issue for their partial carbo-mers, dialkynylbutatriene (DAB) and di(alkynylbutatrienyl)-acetylene (DBA, i.e. the functionality of carbo-n-butadiene) components, was less obvious (Fig. 1).6 Recently, generic acyclic DBA derivatives B were shown to be actually quite stable and could be studied in a systematic manner.7 In passing, they were found to be much more sensitive than the carbo-benzene counterparts A to the effects of electro-active substituents on the maximum UV-vis absorption wavelength (Fig. 1: R = 4-X–C6H4, X = NO2, CF3, H, OMe, NR′2…).7b Whereas the phenomenon was tentatively attributed to macrocyclic aromaticity (“macro-aromaticity”) making the C18 ring of A quite π-independent from its substituents R, the same carbo-benzene ring, which is anyway three-times smaller and three-times less energetically aromatic than benzene,3f has at most a weak electrical insulating effect. Very recently, indeed, the single molecule conductance (SMC) of a functional carbo-benzene A (R = 4-NH2–C6H4)4 measured by STM techniques proved to be much higher than that of benzenoid or porphyrine parents of similar size (ca. 2 nm), and almost two orders of magnitude higher than the SMC of the acyclic DBA counterpart B (R = 4-NH2–C6H4) (106 nS vs. 2.7 nS).8 On the basis of NEGF-DFT-calculations, this SMC difference was correlated with the difference in conformational freedom between the rigid carbo-benzene A and the freely rotating DBA derivative B (Fig. 1). The stiffness of A is, however, also effective in the σ-cyclic and π-acyclic carbo-cyclohexadiene C, which is a rigid version of the non-macro-aromatic DBA B, thus locked in a cisoid conformation. Access to C (R = 4-NH2–C6H4) would thus allow an appraisal of the role of the macro-aromaticity of the equally rigid parent A on conduction. More fundamentally, the carbo-cyclohexadiene C is also the closest realistic non-aromatic but cyclic reference for the carbo-benzene A, just as cyclohexadiene is for benzene.9 | ||
| Fig. 1 The common DBA moiety in three types of carbo-meric molecules related to the carbo-benzene A, generated by reduction of the hexaoxy-[6]pericyclyne D, via the carbo-cyclohexadiene C. A complete A, B, C series is known for R = 4-MeO–C6H4.10 | ||
To date, a single example of carbo-cyclohexadiene has been reported:10aC (R = 4-MeO–C6H4) was incidentally isolated in low yield as a sub-reduced side product of the reductive aromatization of a hexaoxy-[6]pericyclyne10bD to the corresponding carbo-benzene A. The latter was also obtained by prolonged reductive treatment of the parent C (Fig. 1). Though it is quite sensitive in the solid state, this first carbo-cyclohexadiene was found to exhibit a persistent sharp turquoise blue-purple dichromism (or dichromatism) in solution.10a It thus appears in different colors to the human eye depending on the length of the optical path crossing the solution,11 which is an unusual physio-optical property giving a further attractiveness to the class of chromophores C.
In order to guarantee the selective and systematic access to carbo-cyclohexadiene C with various types of substituents R, the control of the reduction step to preserve one of the 1,4-dioxybut-2-yne edges of the precursors D is the synthetic challenge addressed below.
Results and discussion
The sole known carbo-cyclohexadiene C (R = 4-MeO–C6H4) was obtained by serendipity at low temperature, thus indicating that the formation of the two first butatrienic edges of the carbo-benzene target A (R = 4-MeO–C6H4) was slightly faster than the formation of the third one.10 Assuming that the mechanism of action of the reducing system SnCl2/HCl starts with the formation of a bispropargylic carbenium from the corresponding carbinoxy vertex of the hexaoxy-[6]pericyclyne precursor D (Fig. 1),12 the two anisyl-stabilized carbenium centers are likely to initially drive the formation of the butatrienic edges that are conjugated with the anisyl substituents R, as found in C. The phenyl-substituted carbinol ether vertices, though less reactive, remain, however, prone to dissociate under the operating acidic conditions, thus leading to A and making the partial reduction to C difficult to control.10a,12 The selectivity for C should, however, be improved by increasing the difference in the mesomeric donor stabilization (+M effect) of the two types of carbenium centers. Ultimately, this should be improved by deliberately changing the two phenyl substituents for substituents exerting opposite mesomeric or inductive acceptor destabilization (–M or –I effect; the more or less protecting groups are denoted as PG in Scheme 1). Within this prospect, trifluoromethyl groups are ideal candidates:13 it was indeed observed that the quite general method for the conversion of 1,4-dioxybut-2-yne derivatives to the corresponding butatrienes by treatment with SnCl2/HCl is not compatible with CF3 substituents.6c Two substituents PG = CF3 at adjacent carbinoxy vertices of a hexaoxy-[6]pericyclyne DF are therefore anticipated to freeze the reactivity of corresponding 1,4-dioxybut-2-yne edge towards reduction and thus optimize the selectivity for the partly reduced carbo-cyclohexadiene product CFvs. the putative carbo-benzene AF (Scheme 1).The envisaged [8F + 10] and [8 + 10F] strategies to synthesise the bis-trifluoromethylated pericyclynic precursors DF are inspired from a strategy previously used in the tetraphenylated series D and are based on a [8 + 10] cyclization step between a C8 dinucleophile and a C10 dielectrophile, where the index F here refers to bis-trifluoromethylated synthons (Scheme 1). In spite of the recognized specificity of the chemistry of organofluorine compounds, both in terms of reactivity (due to extreme electronegativity and hardness) and purification (due to a high lipophilic character), the general strategic principles developed in the 1,4-diphenylbut-2-yne series10a are shown to be adaptable for the 1,4-bis(trifluoromethyl)but-2-yne series.
1. [8 + 10F] cyclization route to bis-trifluoromethylated hexaoxy-[6]pericyclynes
The fluorinated dialdehyde or diynone synthon C10F was targeted through the known triyne intermediate 1 (Scheme 2).6c The latter was prepared in two steps from the diol 2via the silylated triyne 3, in 75% overall yield (in spite of its volatility) as a statistical mixture of dl and meso diastereoisomers, identified by two 19F NMR singlet signals at −79.52 and −79.53 ppm. The mixture was not resolved before use as either the precursor of the C10F synthon here, or the C8F synthon in the [8F + 10] route (see Section 2). | ||
| Scheme 2 Synthesis of the dicarbonyl synthons C10F, 5a–c, via the bis-trifluoromethylated triyne 1, also serving as the C8F synthon (see Fig. 1). | ||
Three C10F synthons were prepared in two steps, starting with the addition of the dilithium salt of 1 to p-formaldehyde, p-anisaldehyde or benzaldehyde, giving the respective diols 4a, 4b, 4c in 60–93% yields. Subsequent oxidation gave the dialdehyde 5a or diketones 5b or 5c, using either MnO2 in dichloromethane (DCM) at room temperature or IBX in refluxing 1,2-dichloroethane (Scheme 2).
The C10F synthons 5a–c were then treated with the known dilithiated C8 bis-terminal triyne 6 in THF at low temperature under quite diluted conditions (Scheme 3).14 While the bis-tertiary [6]pericyclynediols 7b and 7c were obtained from the corresponding diketones in 40 and 18% yield, respectively, the bis-secondary [6]pericyclynediol 7a could not be obtained from the dialdehyde 5a (the final reaction mixture contained the starting triyne 6 and traces of linear oligomers). The CF3 substituents, replacing the original phenyl substituents,10a are therefore responsible for the uncontrolled reactivity of the carbaldehyde groups in the γ position. The [6]pericyclynediol 7a was also targeted as a possible precursor of the [6]pericyclynedione 8, a putative pivotal reactant for the preparation of 1,10-disubstituted carbo-cyclohexadienes by addition of various nucleophiles to its keto groups (Scheme 3). A similar approach indeed proved to be efficient in the tetraphenyl series for the synthesis of carbo-benzenes through the tetraphenyl-[6]pericyclynedione analogue of 8.4,10a,14b
The synthesis of 7a was also attempted through the alternative [8F + 10] strategy from the fluorinated triyne 1 as the C8F dinucleophile, and the known dialdehyde 9 as the C10 dielectrophile,14b but without more success (Scheme 3).
2. [8F + 10] cyclization route to bis-trifluoromethylated hexaoxy-[6]pericyclynes
In spite of the intriguing failure of the [8F + 10] strategy from the C10 dialdehyde 9 (Section 1, Scheme 3), the same strategy was envisaged from C10 diketones. The triynediones 10d–h were thus prepared using two alternative methods involving the bis-secondary diols 11d–h as intermediates (Scheme 4). The first method, recently described and consisting of a double addition of the bis-terminal triyne 6 to two equivalents of 4-(trifluoromethyl)benzaldehyde, gave the diol 11d in 96% yield.7b The second method, involving the triynedial 9 as a dielectrophile towards various nucleophiles, led to the diols 11e–h. The procedure, previously described for the preparation of 11g in 96% yield from lithium triisopropylsilylacetylide,4 was thus generalized to other nucleophiles, giving the diols 11e,f,h in 27–72 % yield (Scheme 4). Isolation of the indolyl- and carbazolyl-substituted products 11e,f required the aqueous treatment of the reaction medium at low temperature. The diols 11d–h were then oxidized to the corresponding diketones 10d–h using MnO2 in DCM (Scheme 4). | ||
| Scheme 4 Synthesis of the C10 diketone synthons to be involved in a [8F + 10] cyclization route to bis-trifluoromethylated [6]pericyclynes of type DF (see Schemes 1 and 5). | ||
The five C10 diketones 10d–h were then involved in an [8F + 10] cyclization process with the same bis-trifluoromethylated dinucleophile 1, the dilithium salt of which was prepared from either base, n-butyllithium or lithium hexamethyldisilazane (LiHMDS) (Scheme 5). In comparison to the use of a stoichiometric amount of n-butyllithium, which turned out to be inefficient in a few cases, the alternative use of four equivalents of LiHMDS afforded 7e,f,h. While 7e proved to be elusive upon chromatography, the [6]pericyclynediols 7d,f–h were finally obtained in 13–38% yields, i.e. in the classical range of related [8 + 10] cyclization processes.14b,15
 | ||
| Scheme 5 [8F + 10] cyclization strategy from triynediones 10 to bis-trifluoromethylated hexaoxy-[6]pericyclynes 7 of type DF (Scheme 3). | ||
The [6]pericyclynediols 7d–h were obtained as mixtures of diastereoisomers (20 in theory): this was evidenced by the extended ranges of resolved 1H NMR signals of C*(R)OCH3 vertices (R = Ph, CF3) and resolved 19F NMR signals of C*(OMe)CF3 vertices (around −79 ppm), induced by the rigid (cyclic) close stereochemical environment (in contrast, the 4-CF3-C6H4 substituents of 7d, remote from the stereogenic centers, resonate as a single broad 19F singlet at −62.7 ppm: see Fig. 2).
 | ||
| Fig. 2 19F NMR spectrum of the [6]pericyclynediol 7d evidencing the occurrence of a diastereoisomeric mixture, 20 diastereoisomers in theory (CDCl3, 282 MHz). | ||
Although the preparation of 7d–h by the alternative [8 + 10F] route was not attempted, systematic comparison of the two routes will deserve special attention, in particular in view of elucidating the failure of both routes for the target 7a and to allow the design of a suitable procedure for this target.
3. Reduction of bis-trifluoromethylated hexaoxy-[6]pericyclynes to carbo-cyclohexadienes
Reductive treatment of the [6]pericyclynediols 7b–h with SnCl2/HCl afforded the carbo-cyclohexadienes 12b–h (Scheme 6). While the tetraaryl targets 12b–f were readily obtained under classical conditions, the dialkynyl counterparts 12g,h were more elusive. The reduction of 7g was not selective, giving a mixture of undetermined products from which a minute quantity of 12g could be obtained. The reduction of 7h turned out to be selective (with one main spot observed on TLC plates), but the product 12h proved to be unstable in the solid state, giving instantly a black insoluble material when concentrated to dryness. The carbo-cyclohexadiene 12h could, however, be characterized in solution, using a procedure avoiding the complete evaporation of the solvent. | ||
| Scheme 6 The selective four-electron reduction of bis-trifluoromethylated hexaoxy-[6]pericyclynes to the corresponding conjugated carbo-cyclohexadienes. | ||
The diastereoselectivity of the partial reduction process could not be determined from 1H or 19F NMR spectra of the crude materials because of a low resolution, likely due to traces of SnCl2. After chromatography, however, the carbo-cyclohexadienes 12b and 12d–h were obtained as mixtures of meso (cis) and D/L (trans) isomers, identified by pairs of sharp 1H NMR signals for the OCH3 groups and pairs of sharp 19F NMR signals for the CF3 groups directly connected to the C18 macrocycle. For the tetraphenylated carbo-cyclohexadiene 12c, single slightly broadened OC1H3 and C19F3 NMR signals were observed. In all cases, except in the case of 12h (and, perhaps, 12c), the mixture could be resolved by silicagel chromatography. 1H and 19F spectra of the two isomers of the representative example 12d (without assignment) are shown in Fig. 3.
 | ||
| Fig. 3 1H NMR (300 MHz, left) and 19F (282 MHz, right) NMR spectra of the resolved meso (cis) and D/L (trans) diastereoisomers of 12d. Top: less polar (on TLC); bottom: more polar. | ||
4. X-Ray crystallography of carbo-cyclohexadienes
Three of the bis-trifluoromethylated carbo-cyclohexadienes were obtained as crystalline solids.16 X-Ray diffraction (XRD) analyses of selected crystals of 12b, 12c and 12d confirmed the conjugated structure of the carbo-cyclohexadiene core. On the basis of experimental spectroscopic data only, it was not possible a priori to decipher whether the structure of the previously isolated tetraphenylated carbo-cyclohexadiene product was C (R = 4-MeO–C6H4), i.e. the core carbo-mer of the 1,3-cyclohexadiene parent, instead of the core carbo-mer of the 1,4-cyclohexadiene isomer (Fig. 1).10a The assignment was proposed on the basis of a comparison of the experimental UV-vis spectrum with the theoretical spectra of both the regioisomers, calculated at semi-empirical ZINDO or TD-DFT levels (only the conjugated butatriene edges of C give rise to the observed two intense bands spectrum: see Section 5).10a Since 12b exhibits the same two-band UV-vis pattern, the XRD data of 12b confirms the original assignment to C (R = 4-MeO–C6H4).XRD analysis also revealed that the three carbo-cyclohexadiene crystals correspond to the meso (cis) stereoisomers 12b–d, with similar geometrical features (Fig. 4). The C18 macrocycle is slightly distorted (maximum deviation from the mean plane: 0.43 Å for 12b, 0.55 Å for 12c, 0.23 Å for 12d), with small torsion angles of the endocyclic DBA motif: 5.8°, 7.4° and 2.1° in 12b, 12c and 12d, respectively. Nevertheless, in contrast to the quasi-planar carbo-benzenes of type A (Fig. 1), the butatriene and but-2-yne edges of the carbo-cyclohexadienes 12b–d of type CF are mesomerically non-equivalent and exhibit bond lengths close to those reported for the linear (σ-acyclic) DBA analogues of type B.7 Indexing the DBA motif of 12b–d as C7![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C8
C8![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C9
C9![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C10(Ph)–C11
C10(Ph)–C11![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C12–C13(Ph)
C12–C13(Ph)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C14
C14![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C15
C15![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C16, the sequence of the bond lengths from C7 to C12 and from C16 to C11 reads (in Å): 1.355 (±0.010), 1.241 (±0.010), 1.356 (±0.010), 1.423 (±0.005), 1.196 (±0.005). The central and lateral Csp-Csp bonds exhibiting a significant difference in length (Δ = 0.045 Å) are therefore assigned to fixed triple and double bonds, respectively.
C16, the sequence of the bond lengths from C7 to C12 and from C16 to C11 reads (in Å): 1.355 (±0.010), 1.241 (±0.010), 1.356 (±0.010), 1.423 (±0.005), 1.196 (±0.005). The central and lateral Csp-Csp bonds exhibiting a significant difference in length (Δ = 0.045 Å) are therefore assigned to fixed triple and double bonds, respectively.
 | ||
| Fig. 4 Molecular views of the X-ray crystal structures of the carbo-cyclohexadienes 12b (left), 12c (middle), and 12d (right) (Scheme 6). 50% probability level for the thermal ellipsoids. For clarity, all hydrogen atoms, disordered atoms and solvent molecules are omitted. DBA motifs: C7–C16. | ||
5. Absorption spectroscopy of carbo-cyclohexadienes
The carbo-cyclohexadienes 12 are highly chromophoric, possibly giving intense red (12b,c), blue (12d–f,h) or purple (12g) solutions in usual organic solvents. The electronic spectra of 12b–f,h in diluted chloroform solutions are combined in Fig. 5 (the minute quantities and moderate stability of 12g prevented full characterization). The spectra exhibit similar patterns, with two intense bands in the visible region (and a third intense band in the UV region due to carbazole and indole substituents for 12e and 12f, respectively). The same two-band pattern was previously observed for the tetraphenylated analogue C (R = 4-MeO–C6H4), theoretical spectra of which were also calculated at the TDDFT and ZINDO levels.10a These calculations were found to reproduce the observations, with high accuracy for the absolute transition energies (433 and 615 nm at the ZINDO level, vs. 437 and 602 nm for the experimental values) and definite agreement for the relative oscillator strengths (f = 1.60 at 433 nm, and f = 0.77 at 615 nm at the ZINDO level). The transitions were shown to involve the four orbitals of the Gouterman model (HOMO-1, HOMO, LUMO, LUMO+1), and one-electron excitations from the ground state S0 to the first and third excited states S1 and S3, both centered on the conjugated DBA core.10a As the Ph or CF3 substituents at the sp3 carbon atoms of the carbo-cyclohexadiene ring are not π-conjugated with the DBA motif, the same interpretation can be inferred to apply in the CF series. The variation of the maximum absorption wavelength (λmax) as a function of the substituents follows the same trend as the one previously observed for the σ-acyclic DBA derivatives of type B, with a slight general bathochromic shift for the present σ-cyclic series of type CF (Fig. 1).4,7 In this series, and by reference to the tetraphenylated dye 12c, the largest bathochromic shifts are thus observed for the most donating anilinyl-type substituents of 12e and 12f. The still π-donating anisyl substituents of 12b and extended phenylethynyl substituents of 12h also induce higher λmax values than the phenyl substituents of 12c. In contrast, the electron-withdrawing p-trifluoromethylphenyl substituents of 12d induce a small hypsochromic shift below the reference value of 12c at 574 nm.In the cyclic series CF, the molar extinction coefficient was found to vary from 9900 to 112900 L mol−1 cm−1, the limit values being achieved for 12f and 12d, respectively. These values are in the same range as those previously reported in the acyclic series B.7b The fluorophore-substituted carbo-cyclohexadienes 12e and 12f were also found to be emissive at 427 (λexc = 243 nm) and 485 nm (λexc = 348 nm), respectively, namely at lower wavelengths than their σ-acyclic parents in the B series, emitting at around 500 nm.4,7b
Possible solvato-chromism in the carbo-cyclohexadiene series CF was finally investigated for the selected tetrakistrifluoromethylated representative 12d, which, contrary to its congeners, is soluble in a typical range of aprotic solvents, including pentane (see ESI†). Starting from pentane, after a first step of ca 10 nm, a quasi-negligible bathochromic shift with dielectric constant (εr) is observed, followed by another step of 4 nm for toluene, an aromatic solvent prone to bind with 12b through specific π–π-stacking interactions: 416 and 568 nm in pentane (εr = 1.8), 423 and 578 nm in chloroform (εr = 4.8), 424 and 578 nm in THF (εr = 7.5), 427 and 582 nm in toluene (εr = 2.4).
In a related context, as previously reported for a tetraphenyl-carbo-cyclohexadiene of type C,10a the bis-trifluoromethylated dyes of type CF appear more or less dichromic in diluted solution.11 In particular, the dianisyl-carbo-cyclohexadienes of types C (R = 4-MeO–C6H4) and CF (12b) exhibit the same turquoise-blue/deep purple dichromism,11 and almost superimposable UV-vis spectra, with two intense absorption bands at 437 and 602 nm for the C version, and at 442 and 605 nm for the CF version 12b (Fig. 6).
 | ||
| Fig. 6 UV-vis absorption spectra in CHCl3 (bottom) of all the known dianisyl-substituted carbo-meric cores of the types A, B, C (Fig. 1, for R = 4-MeO–C6H4) and CF (= 12b) (top). | ||
The dianisyl series (Fig. 1, R = 4-MeO–C6H4) is completed by two additional representatives A and B, where the central core is a rigid aromatic carbo-benzene ring in A and a flexible σ-acyclic carbo-n-butadiene unit in B. The latter possesses formally the same DBA π-conjugated system as in the σ-cyclic versions C or CF (12b) and exhibits also a two-band absorption spectrum, with similar absorption wavelengths at 443 and 591 nm but with markedly different respective intensities (these intensities are similar in C and CF). This difference is attributed to the much greater flexibility of the DBA motif in B by comparison to C and CF. TDD-DFT or ZINDO calculations of the absorption spectra in the free-rotating series B would thus be much more challenging than in the locked series C, CF or A (see above),10a as they would require full conformational analysis before relevant averaging. The effect of the cisoid-locked conformation of the DBA motif in C and CF (12b) is thus dramatic, resulting, in particular, in a much weaker dichromism of the freely rotating carbo-n-butadiene B.
In contrast to the B, C and CF representatives, the electronic spectrum of the quadrupolar dianisyl carbo-benzene A exhibits only one main absorption band (at 482 nm), as widely documented in the general carbo-benzene series.2c,4,10a
6. Electrochemistry of carbo-cyclohexadienes
The electrochemical properties of the carbo-cyclohexadienes of type CF (excluding the poorly stable dialkynyl derivatives 12g and 12h) were investigated by square-wave (SW) and cyclic voltammetry (CV). The corresponding data are summarized in Table 1.| Compound | Reductions | Oxidations | ||||||
|---|---|---|---|---|---|---|---|---|
| First reduction C series | First reduction B series4,7b | Other reductions C seriesd | First oxidation C series | First oxidation B series4,7b | Other oxidations C seriesd | |||
| E 1/2 | RIpc | E 1/2 | E redp | E 1/2 | RIpc | E 1/2 (ref. 4 and 7b) | E redp | |
| (ΔEp)b | (ΔEp)b | |||||||
| a Half wave potential E1/2 = (Eredp + Eoxp)/2, in V/SCE. b Separation between the two peak potentials: ΔEp = |Eredp – Eoxp|, in V. c Peak current ratio RIp = |Ioxp/Iredp|. d Irreversible unless otherwise noted. e E p values measured from CV in V/SCE. f Scan rate: 0.1 V s−1. g Scan rate: 0.5 V s−1. h After the first oxidation, a product deposited on the electrode. i Formation of an electroactive deposit observed. j Potentials obtained from SWV voltammograms. k Reversible couple: E1/2 = −0.69 V/SCE, ΔEp = 0.07 V, RIp = 0.92. l Shoulder of low intensity, which could possibly correspond to an adsorbed product. | ||||||||
| 12b | −0.67 | 1.00 | −0.88 | −0.95 | 1.12 | 0.86 | 0.95 | 1.68h |
| (0.06)f | −1.18 | (0.09)g | 1.90 | |||||
| −1.44j | ||||||||
| −1.52j | ||||||||
| −1.61j | ||||||||
| 12c | −0.61 | 0.94 | −0.80 | −0.88 | 1.33 | 1.05 | 0.95 | 1.60g |
| (0.06) | −1.07 | (0.06) | ||||||
| −1.57i | ||||||||
| 12d | −0.47 | 0.96 | −0.65 | −0.69k | 1.54h | — | 1.31 | 1.86 |
| (0.06) | −1.84 | irr. | — | |||||
| 12e | −0.59irr. | — | −0.75 | −0.80l | 1.21h,i | — | 1.06 | 1.42 |
| −1.00 | irr. | 1.68 | ||||||
| −1.44j | ||||||||
| −1.73j | ||||||||
| 12f | −0.60 | 1.17 | −0.75 | −0.85 | 1.17h,i | — | 1.04 | 1.60 |
| (0.06) | −1.00 | irr. | ||||||
| −1.43j | ||||||||
| −1.74j | ||||||||
All the carbo-cyclohexadienes exhibit quasi-identical reduction behaviour, with three waves. The first one is reversible (except for 12e) and occurs between −0.47 and – 0.67 V (the latter limit values being achieved for 12d and 12b, respectively). As previously observed in the carbo-n-butadiene series B, and as expected,7b the acceptor-substituted derivative 12d of the CF series (R = 4-CF3–C6H4) is thus more prone to reduction. The first reduction potentials are, however, systematically higher (in algebraic value) in the carbo-cyclohexadiene series CF than in the carbo-n-butadiene series B (italicized values in Table 1), likely because of the greater average stabilization of the anion through the optimally conjugated quasi-planar DBA motif of the CF series. Another similarity between the series CF and B is the result of the less donating character of the indolylphenyl and carbazolylphenyl substituents with respect to the anisyl substituent, the latter giving the smallest half-wave potential of the carbo-cyclohexadiene series (E1/2 = −0.67 V for 12b).
In the oxidation process, the cations of the indolylphenyl- and carbazolylphenyl- substituted carbo-cyclohexadienes 12e and 12f were found to deposit on the electrode, giving electroactive films. In a complementary manner to what is observed in the reduction process, the carbo-cyclohexadienes CF are less prone to oxidation than the carbo-n-butadiene counterparts B (italicized values in Table 1). Only the first oxidation waves of the dianisyl- and diphenyl-substituted derivatives 12b and 12c are reversible, with the most π-donating group, R = 4-MeO–C6H4, giving the lowest potential of the series (E1/2 = +1.12 V for 12b). In contrast, the most electron-withdrawing group, R = 4-CF3–C6H4, induces the highest first oxidation potential at 1.54 V for 12d. The quite high first oxidation potentials in the CF series make the results difficult to interpret (due to the close oxidation wave of the solvent), but can be correlated to the corresponding low first reduction potentials: following a general trend, molecules that are readily reduced are not easily oxidized.
Conclusion
Since the incidental isolation of the first example of carbo-cyclohexadiene resulting from a partial reduction of a [6]pericyclyne,10a the introduction of a trifluoromethyl group on two adjacent vertices of hexaoxy-[6]pericyclynes allowed the selective synthesis of conjugated carbo-cyclohexadienes. These bore various types of electroactive substituents at the 1,10 positions of the endo-macrocyclic DBA motif, with spectator phenyl substituents at the 4,7 positions. Whereas the tetraaryl bis-trifluoromethylated carbo-cyclohexadienes were found to be stable both in solution and in the solid state, two dialkynyldiphenyl counterparts appeared less stable. Moreover, the trifluoromethylated carbo-cyclohexadienes appear much more stable than their phenylated analogues, without modifying their chromophoric and spectroscopic properties, as evidenced in the anisyl-substituted series. The optical and electronic properties of this novel type of carbo-meric chromophore deserve systematic attention. In particular, as justified in the introduction, the dianilinyl derivative CF (R = 4-NH2–C6H4, Fig. 1) is currently being targeted for measurement of its single molecule conductance (SMC) and comparison with the carbo-benzene A (R = 4-NH2–C6H4).8 As neither the [8 + 10F] nor the [8F + 10] strategy proved to be compatible with the NH2 substituents, further methodological improvements are required. In parallel, theoretical studies are being undertaken to bring out the specific, but subtle effects of the CF3 substituents in the CF series, with respect to the phenyl substituents in the C series.10a,17Acknowledgements
I. B. thanks the French Embassy in Kiev, Ukraine, for financial support. In addition to the GDRI “groupement franco-ukrainien en chimie moléculaire” funded by the Centre National de la Recherche Scientifique (CNRS), the ANR program (ANR-11-BS07-016-01) is acknowledged for the post-doctoral fellowship of A.R. Thanks are finally due to Dr Evelyne Chelain for her valuable advice on fluorine chemistry, and to Mr Kévin Cocq for his assistance in studying solvent effects in UV-vis absorption spectroscopy.Notes and references
- (a) R. Chauvin, Tetrahedron Lett., 1995, 36, 397–400 CrossRef CAS; (b) V. Maraval and R. Chauvin, Chem. Rev., 2006, 106, 5317–5343 CrossRef CAS PubMed.
- For experimental syntheses of carbo-benzenes, see for example: (a) Y. Kuwatani, N. Watanabe and I. Ueda, Tetrahedron Lett., 1995, 36, 119–122 CrossRef CAS; (b) R. Suzuki, H. Tsukude, N. Watanabe, Y. Kuwatani and I. Ueda, Tetrahedron, 1998, 54, 2477–2496 CrossRef CAS; (c) C. Saccavini, C. Sui-Seng, L. Maurette, C. Lepetit, S. Soula, C. Zou, B. Donnadieu and R. Chauvin, Chem.–Eur. J., 2007, 13, 4914–4931 CrossRef CAS PubMed; (d) C. Zou, C. Duhayon, V. Maraval and R. Chauvin, Angew. Chem., Int. Ed., 2007, 46, 4337–4341 CrossRef CAS PubMed; (e) I. Baglai, V. Maraval, C. Bijani, N. Saffon-Merceron, Z. Voitenko, Y. M. Volovenko and R. Chauvin, Chem. Commun., 2013, 49, 8374–8837 RSC.
- For theoretical studies on the aromaticity of carbo-benzenes, see: (a) C. Godard, C. Lepetit and R. Chauvin, Chem. Commun., 2000, 1833–1834 RSC; (b) C. Lepetit, C. Godard and R. Chauvin, New J. Chem., 2001, 25, 572–580 RSC; (c) C. Lepetit, B. Silvi and R. Chauvin, J. Phys. Chem. A, 2003, 107, 464–473 CrossRef CAS; (d) C. Zou, C. Lepetit, Y. Coppel and R. Chauvin, Pure Appl. Chem., 2006, 78, 791–811 CrossRef CAS; (e) C. Lepetit, C. Zou and R. Chauvin, J. Org. Chem., 2006, 71, 6317–6324 CrossRef CAS PubMed; (f) R. Chauvin, C. Lepetit, V. Maraval and L. Leroyer, Pure Appl. Chem., 2010, 82, 769–800 CrossRef CAS; (g) J.-M. Ducéré, C. Lepetit and R. Chauvin, J. Phys. Chem. C, 2013, 117, 21671–21681 CrossRef.
- A. Rives, I. Baglai, V. Malytskiy, V. Maraval, N. Saffon-Merceron, Z. V. Voitenko and R. Chauvin, Chem. Commun., 2012, 48, 8763–8765 RSC.
- The “double-cut aromatic cyclic energy”, ACEDC, is a rationally devised approximate of the exact topological aromaticity of a ring; for benzene, it is the enthalpy of the homodesmotic equation: 2 1,3,5-n-hexatriene = benzene + ethylene + 1,3-butadiene. See: (a) J.-P. Malrieu, C. Lepetit, M. Gicquel, J.-L. Heully, P. W. Fowler and R. Chauvin, New J. Chem., 2007, 31, 1918–1927 RSC.
- (a) J.-D. van Loon, P. Seiler and F. Diederich, Angew. Chem., Int. Ed. Engl., 1993, 32, 1187–1189 CrossRef; (b) A. Auffrant, B. Jaun, P. D. Jarowski, K. N. Houk and F. Diederich, Chem.–Eur. J., 2004, 10, 2906–2911 CrossRef CAS PubMed; (c) V. Maraval, L. Leroyer, A. Harano, C. Barthes, A. Saquet, C. Duhayon, T. Shinmyozu and R. Chauvin, Chem.–Eur. J., 2011, 17, 5086–5100 CrossRef CAS PubMed.
- (a) A. Rives, V. Maraval, N. Saffon-Merceron and R. Chauvin, Chem.–Eur. J., 2012, 18, 14702–14707 CrossRef CAS PubMed; (b) A. Rives, V. Maraval, N. Saffon-Merceron and R. Chauvin, Chem.–Eur. J., 2014, 20, 483–492 CrossRef CAS PubMed.
- Z. Li, M. Smeu, A. Rives, V. Maraval, R. Chauvin, M. A. Ratner and E. Borguet, submitted.
- Since the pioneering work of L. P. Hammett (L. P. Hammett, Physical Organic Chemistry, McGraw-Hill, NewYork, 1940), the effects of substituents on benzene derivatives have been thoroughly investigated, in particular by comparison with cyclohexadienes, which were theoretically evidenced to be more sensitive to substituent effects than their benzenic counterparts: (a) T. M. Krygowski and B. T. Stępień, Chem. Rev., 2005, 105, 3482–3512 CrossRef CAS PubMed; (b) T. M. Krygowski, M. A. Dobrowolski, M. K. Cyrański, W. P. Oziminski and P. Bultinck, Comput. Theor. Chem., 2012, 984, 36–42 CrossRef CAS.
- (a) L. Leroyer, C. Lepetit, A. Rives, V. Maraval, N. Saffon-Merceron, D. Kandaskalov, D. Kieffer and R. Chauvin, Chem.–Eur. J., 2012, 18, 3226–3240 CrossRef CAS PubMed; (b) original reference on [6]pericyclynes: L. T. Scott, G. J. DeCicco, J. L. Hyun and G. Reinhardt, J. Am. Chem. Soc., 1985, 107, 6546–6555 CrossRef CAS.
- Dichromism, or dichromatism, is a property of some materials or solutions, which makes them appear in two different colors to the human eye depending on the concentration of the absorbing dye and on the thickness of the traversed medium. For references, see: (a) H. Cartwright, J. Chem. Educ., 1986, 63, 984–987 CrossRef CAS; (b) S. Kreft and M. Kreft, Naturwissenschaften, 2007, 94, 935–939 CrossRef CAS PubMed; (c) J. P. Launay, unpublished report, Toulouse (private communication); (d) J. P. Launay, La vision des couleurs, “Regards Croisés” conference at the Interdisciplinary Doctoral Workshop 2014-2015 of the IUF on the theme “The color”, University of Toulouse, November 4, 2014.
- L. Leroyer, V. Maraval and R. Chauvin, Chem. Rev., 2012, 112, 1310–1343 CrossRef CAS PubMed.
- See for example: (a) A. D. Allen and T. T. Tidwell, in Advances in Carbocation Chemistry, ed. X. Creary, JAI Press, Greenwich, 1989, vol. 1, pp. 1–44 Search PubMed; (b) T. Sürig, H.-Fr. Grützmacher, J.-P. Bégué and D. Bonnet-Delpon, Org. Mass Spectrom., 1993, 28, 254–261 CrossRef; (c) K. K. Laali, M. Tanaka, S. Hollenstein and M. Cheng, J. Org. Chem., 1997, 62, 7752–7757 CrossRef CAS; (d) M. Gruselle, B. Malézieux, R. Andrés, H. Amouri, J. Vaissermann and G. G. Melikyan, Eur. J. Inorg. Chem., 2000, 359–368 CrossRef CAS; (e) J. P. Richard, T. L. Amyes and M. M. Toteva, Acc. Chem. Res., 2001, 34, 981–988 CrossRef CAS PubMed.
- (a) L. Maurette, C. Tedeschi, E. Sermot, M. Soleilhavoup, F. Hussain, B. Donnadieu and R. Chauvin, Tetrahedron, 2004, 60, 10077–10098 CrossRef CAS; (b) L. Leroyer, C. Zou, V. Maraval and R. Chauvin, C. R. Chim., 2009, 12, 412–429 CrossRef CAS.
- C. Saccavini, C. Tedeschi, L. Maurette, C. Sui-Seng, C. Zou, M. Soleilhavoup, L. Vendier and R. Chauvin, Chem.–Eur. J., 2007, 13, 4895–4913 CrossRef CAS PubMed.
-
(a) Crystallographic data for 12b: C48H30F6O4, M = 784.75, Monoclinic, space group C2/c, a = 13.07527(8) Å, b = 16.18867(10) Å, c = 19.01027(12) Å, β = 100.1002(6)°, V = 3961.57(4) Å3, Z = 4, crystal size: 0.15 × 0.15 × 0.25 mm3, 35692 reflections collected (3771 independent, Rint = 0.0213), 262 parameters, R1 [I > 2σ(I)] = 0.049, wR2 [all data] = 0.065, largest diff. peak and hole: 0.61 and −0.21 e.Å−3, CCDC 1003439;
(b) crystallographic data for 12c: C48H24F12O2, CHCl3, M = 980.04, Monoclinic, space group P21/c, a = 12.8182(16) Å, b = 35.815(5) Å, c = 9.7626(14) Å, β = 92.841(6)°, V = 4476.4(11) Å3, Z = 4, crystal size: 0.20 × 0.20 × 0.04 mm3, 59064 reflections collected (7551 independent, Rint = 0.1994), 653 parameters, 102 restraints, R1 [I > 2σ(I)] = 0.0829, wR2 [all data] = 0.2414, largest diff. peak and hole: 0.573 and –0.326 e.Å−3, CCDC 951896;
(c) crystallographic data for 12d: C46H26F6O2, CH2Cl2, M = 809.59, Triclinic, P
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , a = 12.5119(17) Å, b = 12.6648(17) Å, c = 14.9565(19) Å, α = 70.805(5)°, β = 66.621(5)°, γ = 67.665(5)°, V = 1967.5(5) Å3, Z = 2, crystal size: 0.20 × 0.10 × 0.04 mm3, 28934 reflections collected (7350 independent, Rint = 0.0906), 669 parameters, 489 restraints, R1 [I > 2σ(I)] = 0.0672, wR2 [all data] = 0.1983, largest diff. peak and hole: 0.212 and –0.314 e.Å−3, CCDC 951897.
, a = 12.5119(17) Å, b = 12.6648(17) Å, c = 14.9565(19) Å, α = 70.805(5)°, β = 66.621(5)°, γ = 67.665(5)°, V = 1967.5(5) Å3, Z = 2, crystal size: 0.20 × 0.10 × 0.04 mm3, 28934 reflections collected (7350 independent, Rint = 0.0906), 669 parameters, 489 restraints, R1 [I > 2σ(I)] = 0.0672, wR2 [all data] = 0.1983, largest diff. peak and hole: 0.212 and –0.314 e.Å−3, CCDC 951897. - H. Hamdani, C. Lepetit and R. Chauvin, unpublished results.
Footnotes |
| † The investigations presented in this report have been performed within the framework of the French-Ukrainian GDRI “Groupement Franco-Ukrainien en Chimie Moléculaire” funded by the CNRS. |
| ‡ Electronic supplementary information (ESI) available: Experimental details, spectroscopic and crystallographic data. CCDC 1003439, 951896 and 951897. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4sc02742f |
| This journal is © The Royal Society of Chemistry 2015 |