Borneol-grafted cellulose for antifungal adhesion and fungal growth inhibition†
Abstract
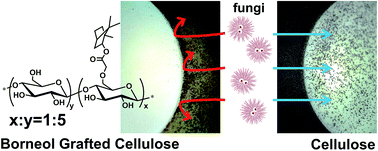
Cellulose fibers modified to obtain antifungal activity have attracted much attention due to their versatile applications, although there are still some problems associated with the conventional protocols, including the toxicity to organisms, unwanted resistance, and gradually increasing environmental pressure. A natural and safe strategy is desired to design new types of antifungal cellulose. Herein, we present a borneol-grafted cellulose (BGC) by covalently tethering L-borneol, a natural product, to cellulose. The attained BGC has been characterized to confirm the chemical and amorphous features using a combination of spectroscopic and analytical techniques. This BGC material was subsequently challenged with Mucor racemosus and Aspergillus niger, and exhibited a remarkable performance in antifungal adhesion and fungal growth inhibition, suggesting the grafted borneol moieties are crucial for influencing the tactile sensing of fungal cells and subsequent selective inadhesion. The BGC has also been evaluated as a non-cytotoxic material, implying its great potential for biomedical and sanitary applications.

 Please wait while we load your content...
Please wait while we load your content...