Low-dose paeonol derivatives alleviate lipid accumulation†
Kuan-Chuan Pao‡
a,
Jin-Feng Zhao‡b,
Tzong-Shyuan Leeb,
Ying-Pei Huangac,
Chien-Chung Hanc,
Lin-Chiang Sherlock Huangac,
Kou-Hung Wua and
Ming-Hua Hsu*a
aNuclear Science & Technology Development Center, National Tsing Hua University, Hsinchu 30013, Taiwan. E-mail: mhhsu@mx.nthu.edu.tw; Fax: +886-3-572-5974; Tel: +886-3-573-1180
bDepartment of Physiology, National Yang-Ming University, Taipei 11221, Taiwan
cDepartment of Chemistry, National Tsing Hua University, Hsinchu 30013, Taiwan
First published on 15th December 2014
Abstract
Here, we present a series of novel paeonol derivatives that prevent lipid accumulation at lower doses and exhibit improved water solubility. According to SAR analysis results, 1-(4-methoxy-2-(2-(piperidin-1-yl)ethoxy)phenyl)ethanone (6a) and 4′-methoxy-2′-[(phenylsulfonyl)oxy]acetophenone (7a) demonstrate as good inhibition ability at low-dose (10 μg mL−1) as that of paeonol at high-dose (100 μg mL−1). In addition, compounds 6a and 7a can be synthesized quantitatively with simple preparation methods. Hence, we believe that compounds 6a and 7a are potentially suitable antiatherogenic compounds for future drug development.
Paeonol 1-(2-hydroxy-4-methoxyphenyl)ethanone (1; Fig. 1) is a phenolic compound isolated from the main active component of Cortex Moutan, a traditional Chinese herbal medicine. Accompanying the considerable volume of scientific research on Chinese herbal medicine,1–7 a growing body of evidence has indicated the benefits of using paeonol to treat various diseases. Paeonol is a widely used antiinflammatory agent for repairing oxidative damage and enhancing immunity function in vivo.8 In lipopolysaccharide-induced macrophages, paeonol can downregulate inflammation-associated genes.9 At specific concentrations, paeonol can prevent oxidized low-density lipoprotein (ox-LDL)-induced monocyte adhesion to vascular endothelial cells by attenuating the expression of vascular cell adhesion molecule-1 and phosphorylation of c-Jun N-terminal and extracellular signal-regulated kinases.10 Moreover, paeonol represses the expression of micro-RNA-21 and release of tumor necrosis factor, which can protect rat vascular endothelial cells from ox-LDL-induced injury.11 Furthermore, paeonol can reduce the severity of liver fibrosis12 and prevent ox-LDL-induced endothelial cell apoptosis.13 Recent studies have indicated that paeonol is potentially suitable for treating various types of cancer. Paeonol enhances apoptosis in ovarian cancer cells by downregulating survivin expression and activating caspase-3 expression.14 Both in vitro and in vivo studies have indicated the radiosensitizing effect of paeonol on lung adenocarcinoma.15 As a preventative Chinese herbal medicine, paeonol can facilitate preventing memory loss following ischemic stroke, and it can improve oral glucose tolerance in patients receiving antidiabetic drugs.16,17 Hence, paeonol is a potentially suitable candidate for further drug development.
Because of the effectiveness of paeonol in treating various types of inflammatory disease, several studies9,18,19 have reported that paeonol can facilitate preventing cardiovascular disease and treating atherosclerosis. Moreover, a previous research18 showed that paeonol effectively mitigated the development of atherosclerosis in animal models. Zhao et al.20 reported that paeonol exhibits a novel effect on the formation of foam cells. Furthermore, it positively attenuates cholesterol accumulation induced by ox-LDL in macrophages, and it can prevent foam cells from forming by increasing ATP-binding membrane cassette transport protein A1 (ABCA1)-dependent cholesterol efflux. Zhao et al. also reported that paeonol enhanced the transcriptional regulation of ABCA1 via activating liver X receptor α (LXRα) activity. According to previous researches,21 the accumulation of lipid-laden foam-cells is characterized by preatherosclerotic lesions, the potential mechanisms of which involve either the uncontrolled uptake of ox-LDL or impaired cholesterol efflux in macrophages. Collectively, the findings of these studies indicate that paeonol is a highly suitable candidate for regulating cholesterol efflux and preventing atherosclerosis.
In a previous study, paeonol treatment markedly alleviated the exacerbation of atherosclerosis in animal models.18 Similar results have been observed in anticancer tests and diabetic prevention trials.15,17 However, a typical dose of these assays is extraordinarily high; for example, radiosensitization of lung adenocarcinoma involves a combination of 1718 mg kg−1 of paeonol and 10 Gy of irradiation.15 In addition, Xiao et al.22 and Xie et al.23 have reported that the distribution of paeonol in the bloodstream ranges from 4 to 10 μg mL−1 after only one dose is orally delivered through rapid absorption.
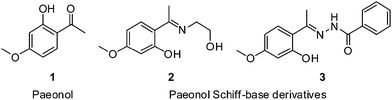
Paeonol and its Schiff base derivatives (2, 3; Fig. 1) can form complexes with copper ions, which exhibits high antioxidant activity, moderate DNA-binding activity, and adequate tumor-cell cytotoxicity.24 The nitrogen atom of the Schiff base contributes to the metal-chelating site and hydrogen bond acceptor in the biological system. Our lab had been study drug development for years, and we found that paeonol modified with phenylsulfonyl groups in the side chain could acquire higher bioactivity.25 This study presents a series of novel paeonol derivatives conjugated with the side chain of the Schiff base, O-alkylation heterocyclic rings and phenylsulfonyl groups, which prevent lipid accumulation at lower doses.
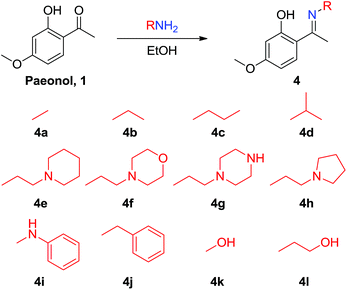
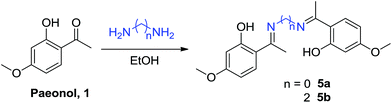
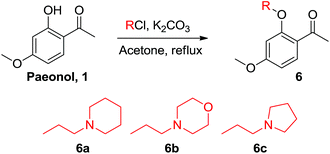
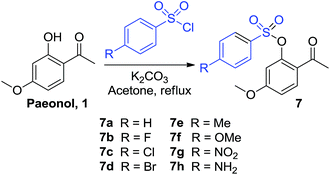
Paeonol is the extract of Paeonia suffruticosa Andr., which has been used in traditional Chinese medicine for thousands of years; however, paeonol exhibits poor water solubility. The paeonol Schiff base derivatives 4a–4l were prepared by condensing paeonol with a series of primary amines bearing heteroatom-substituted amine side chains to increase its solubility and bioactivity (Scheme 1). The nitrogen atom of the Schiff base can improve overall solubility in water molecules, not because nitrogen is hydrophilic but because it can function as a hydrogen bond acceptor. Dimeric paeonol derivatives were obtained by treating paeonol with hydrazine to produce the zero-length derivative 5a or by using ethyldiamine to obtain a Schiff-base-conjugated double-head paeonol derivative 5b (Scheme 2). Furthermore, we synthesized the O-alkylation products of paeonol (6a–6c) through the phenol group of the paeonol by using potassium carbonate as a base and acetone as a solvent under reflux conditions (Scheme 3). We also conjugated the phenylsulfonyl group with paeonol through the phenol group to obtain new series of paeonol derivatives 7a–7h (Scheme 4).
Various paeonol derivatives were prepared using a chemical synthesis technique to generate 14 Schiff-base-conjugated paeonol derivatives, including two double-head conjugated derivatives, 11 O-alkylation products, 3 of which with heterocycle ring and 8 of which bear phenylsulfonyl groups. Compared with natural paeonol, most of the synthesized compounds exhibited improved solubility in water because of the imine group in the core structure. Among these derivatives, the paeonol derivative 4g bearing a piperazine ring (4g) exhibited a maximal solubility of 6.579 mg mL−1 (natural paeonol is 0.524 mg mL−1; see ESI Table S1†). Improved water solubility is beneficial because it might improve pharmacokinetic research and lead to the development of novel drugs. However, some of the new synthesized compounds (4i, 4j, 5, and 7) were more insoluble than natural paeonol, because these compounds contained a hydrophobic aromatic moiety that reduced the overall molecular hydrophilic affinity to the water.
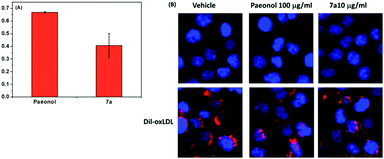
The modified LDL (i.e., ox-LDL) disrupts the metabolism of lipids in macrophages, leading to foam cell formation and the induction of atherosclerosis. Therefore, we performed a fluorescent assay to confirm that the paeonol derivatives can prevent lipid accumulation in macrophages in ox-LDL treatment. Herein, we reported that the series of novel paeonol derivatives exhibited more favorable bioactivity in downregulating lipid accumulation than paeonol (Table 1). We found compounds 4i, 5a, 5b, 7e, and 7g were precipitated in the media when treating with cell due to the poor solubility. Meanwhile, MTT results (see ESI Fig. S1†) revealed that most of the phenylsulfonyl series compounds showed strong cytotoxicity, which caused the cell death. The results in Fig. 2 indicated that some of these agents exerted the same effect as paeonol, but at lower doses (100-fold lower). Lee et al. reported that paeonol clearly influences lipid accumulation (Table 1). This study revealed that preincubation with novel paeonol derivatives exhibited exceptional bioactivity in regulating lipid content. When the doses were identical (100 μg mL−1) for the fluorescent assay, the compounds 4j, 6a, and 7a were substantially more effective in inhibiting lipid accumulation than natural paeonol (Fig. 2). In particular, the compounds 6a and 7a downregulated 60–65% of lipid accumulation, whereas paeonol downregulated only 30%. Moreover, at the high dose test of 100 μg mL−1, the proposed derivatives clearly exhibited potential in reducing the dose requirements. To elucidate the low-dose effect of paeonol derivatives on lipid accumulation, we performed a dose-dependent fluorescent assay (0.1–100 μg mL−1), as shown in Fig. 3. At a dose of 10 μg mL−1, the compounds 4a, 4j, 6a, 6c, and 7a were as effective as a 100 μg mL−1 dose of natural paeonol. In Fig. 4, the low-dose treatment of compound 7a was more effective than natural paeonol administered in a high dose (40% vs., 30%). At a dose of 1 μg mL−1, the natural paeonol was ineffective in preventing lipid accumulation. By contrast, the compounds 4a, 4j, 6c, and 7a exhibited slight bioactivity. Furthermore, some of these compounds exhibited minor effects on lipid accumulation when the dose was reduced 1000-fold (0.1 μg mL−1).
| Compoundsa | Lipid accumulationb | Compoundsa | Lipid accumulationb |
|---|---|---|---|
| a Paeonol derivatives in 100 μg mL−1.b Lipid accumulation in macrophage by fluorescent assay. | |||
| Paeonol | 0.669 ± 0.006 | 5a | Not resolve |
| 4a | 0.656 ± 0.067 | 5b | Not resolve |
| 4b | 0.785 ± 0.007 | 6a | 0.352 ± 0.019 |
| 4c | 0.509 ± 0.07 | 6b | 0.700 ± 0.067 |
| 4d | 0.836 ± 0.242 | 6c | 0.705 ± 0.046 |
| 4e | 0.727 ± 0.033 | 7a | 0.406 ± 0.096 |
| 4f | 0.931 ± 0.175 | 7b | Cell death |
| 4g | 0.782 ± 0.107 | 7c | Cell death |
| 4h | 0.584 ± 0.087 | 7d | Cell death |
| 4i | Not resolve | 7e | Not resolve |
| 4j | 0.464 ± 0.054 | 7f | Cell death |
| 4k | Cell death | 7g | Not resolve |
| 4l | 0.499 ± 0.114 | 7h | Cell death |
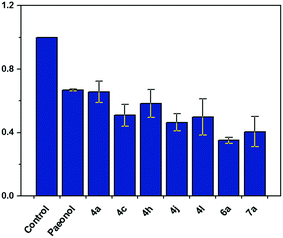 | ||
| Fig. 2 Bio-activity of paeonol derivatives for down-regulated lipid accumulation in macrophages. Data are mean ± S.E.M. from three independent experiments. *P < 0.05 versus paeonol. | ||
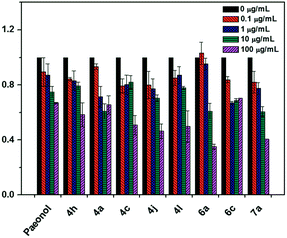 | ||
| Fig. 3 Fold changes of lipid accumulation within paeonol derivatives treatment in indicated concentrations. Data are mean ± S.E.M. from three independent experiments. | ||
The structure–activity relationship (SAR) of the compounds was evaluated based on our results. Among the series of imine-conjugated paeonol derivatives, the compound 4c bearing a propyl group was the most efficacious, compared with those bearing methyl, ethyl, and isopropyl groups. The heterocyclic compounds bearing piperidine, pyrrolidine, morpholine, and piperazine rings (4e–4h, respectively) exhibited increased solubility in water; however, this had no effect on downregulated lipid accumulation in macrophages. The double-head conjugated paeonol derivatives 5a and 5b were insoluble in water and precipitated in media during cell-culture treatment. Compounds 4i and 4j were similar structures bearing phenyl rings with poor water solubility. Although 4j exhibited adequate regulation of lipid accumulation at low doses, 4i caused cell death. Similar phenomena were observed for compounds 4k (cell death) and 4l (adequate regulation). Among the O-alkylation paeonol derivatives, compound 6a was the most potent compound in inhibiting lipid accumulation. The piperidine-modified derivative 6a was more active than either morpholine 6b or pyrrolidine 6c. Furthermore, we found most of the phenylsulfonyl paeonol derivatives cause cell death; however, the 7a exhibited high activity. The SAR analysis suggested that phenylsulfonyl moieties bearing halide (7b, 7c, and 7d), OMe (7f), and NH2 (7h) cause cell death. Meanwhile, the 7c with CH3 group and 7d with NO2 group were too hydrophobic to precipitate in the media. The low-dose treatment of compound 7a achieved the same effect as paeonol administered in 100 μg mL−1 (Fig. 4). The results indicated that substituting the phenol group with a phenylsulfonyl side chain is a new strategy for achieving further chemical modification.
Conclusions
In summary, we synthesized 14 paeonol derivatives containing Schiff-base conjugates and 11 O-alkylation derivatives. The water solubility of the compounds was evaluated, and the results revealed that the compounds bearing a heterocyclic ring exhibited increased solubility in water, although such increase exerted no effect on downregulated lipid accumulation in macrophages. The SAR analysis results indicated that compounds 6a and 7a are potent inhibitors of lipid accumulation at low doses. In accordance with Zhao et al.,20 we hypothesize that this novel series of paeonol derivatives follows the paeonol pathway to regulate lipid accumulation by upregulating the nuclear translocation of LXRα, which enhances the mRNA and protein expression of ABCA1, thereby reducing the accumulation of cholesterol. The results indicated that compounds 6a and 7a are potentially suitable antiatherogenic compounds for future drug development.Notes and references
- S. Akhondzadeh and S. H. Abbasi, Chin. J. Integr. Med., 2006, 21, 113–118 Search PubMed.
- G. G. Yue, K. M. Chan, M. H. To, L. Cheng, K. P. Fung, P. C Leung and C. B. Lau, J. Nat. Prod., 2014, 77, 1074–1077 CrossRef CAS PubMed.
- Q. Zhou, W. Z. Qin, S. B. Liu, J. S. Kwong, J. Zhou and J. Chen, Cochrane Database Syst. Rev., 2014, 4, CD005052, DOI:10.1002/14651858.CD005052.pub5.
- J. L. Ru, P. Li, J. N. Wang, W. Zhou, B. H. Li, C. Huang, P. D. Li, Z. H. Guo, W. Y. Tao, Y. F. Yang, X. Xu, Y. Li, Y. H. Wang and L. Yang, J. Cheminf., 2014, 6, 13 Search PubMed.
- Y. Ueda, K. Mori, S. Satoh, H. Dansako, M. Ikeda and N. Kato, Biochem. Biophys. Res. Commun., 2014, 447, 341–345 CrossRef CAS PubMed.
- Y. C. Chen, F. P. Chen, T. J. Chen, L. F. Chou and S. J. Hwang, Patterns of traditional Chinese medicine use in patients with inflammatory bowel disease: a population study in Taiwan, Hepato-gastroenterology, 2008, 55, 467–470 Search PubMed.
- S. Wang, S. Penchala, S. Prabhu, J. Wang and Y. Huang, Curr. Drug Discovery Technol., 2010, 7, 67–75 CrossRef CAS.
- B. Chen, M. L. Ning and G. S. Yang, Molecules, 2012, 17, 4672–4683 CrossRef CAS PubMed.
- H. Huang, E. J. Chang, Y. Lee, J. S. Kim, S. S. Kang and H. H. Kim, Inflammation Res., 2008, 57, 189–198 CrossRef CAS PubMed.
- Y. Q. Wang, M. Dai, J. C. Zhong and D. K. Yin, Biol. Pharm. Bull., 2012, 35, 767–772 CAS.
- Y. R. Liu, J. J. Chen and M. Dai, Acta Pharmacol. Sin., 2014, 35, 483–488 CrossRef CAS PubMed.
- D. Kong, F. Zhang, D. Wei, X. Zhu, X. Zhang, L. Chen, Y. Lu and S. Zheng, J. Gastroenterol. Hepatol., 2013, 28, 1223–1233 CrossRef CAS PubMed.
- M. H. Bao, Y. W. Zhang and H. H. Zhou, J. Ethnopharmacol., 2013, 146, 543–551 CrossRef CAS PubMed.
- J. Yin, N. S. Wu, F. Q. Zeng, C. Cheng, K. Kang and H. Yang, Acta Histochem., 2013, 115, 835–839 CrossRef CAS PubMed.
- Y. Lei, H. X. Li, W. S. Jin, W. R. Peng, C. J. Zhang, L. J. Bu, Y. Y. Du, T. Ma and G. P. Sun, Int. J. Radiat. Biol., 2013, 89, 1079–1086 CrossRef CAS PubMed.
- S. Yu. Su, C. Yi. Cheng, T. H. Tsai and C. L. Hsieh, Evidence-Based Complementary and Alternative Medicine, 2012, 2012, 932823 Search PubMed.
- C. H. Lau, C. M. Chan, Y. W. Chan, K. M. Lau, T. W. Lau, F. C. Lam, W. T. Law, C. T. Che, P. C. Leung, K. P. Fung, Y. Y. Ho and C. B. S. Lau, Phytomedicine, 2007, 14, 778–784 CrossRef CAS PubMed.
- H. Li, M. Dai and W. Jia, Planta Med., 2009, 75, 7–11 CrossRef CAS PubMed.
- Y. J. Li, J. X. Bao, J. W. Xu, F. Murad and K. Bian, Vasc. Pharmacol., 2010, 53, 169–176 CrossRef CAS PubMed.
- J. F. Zhao, S. J. J. Leu, S. K. Shyue, K. H. Su, J. Wei and T. S. Lee, Am. J. Chin. Med., 2013, 41, 1079–1096 CrossRef PubMed.
- A. C. Li and C. K. Glass, Nat. Med., 2002, 8, 1235–1242 CrossRef CAS PubMed.
- Y. Xiao, Y. H. Zhang, Y. X. Sheng and J. L. Zhang, Biomed. Chromatogr., 2008, 22, 527–534 CrossRef CAS PubMed.
- Y. Xie, H. Zhou, Y. F. Wong, H. X. Xu, Z. H. Jiang and L. Liu, J. Pharm. Biomed. Anal., 2008, 46, 748–756 CrossRef CAS PubMed.
- D.-D. Qin, Z.-Y. Yang, F.-H. Zhang, B. Du, P. Wang and T.-R. Li, Inorg. Chem. Commun., 2010, 13, 727–729 CrossRef CAS PubMed.
- T.-J. Huang, H. Chuang, J.-C. Horng, Y.-C. Kuo, C.-W. Chen, F.-Y. Tsai, Y.-C. Liang, S.-C. Yen, S.-C. Chou and M.-H. Hsu, Eur. J. Med. Chem., 2015, 90, 428–435 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 0.1039/c4ra13986k |
| ‡ Kuan-Chuan Pao and Jin-Feng Zhao contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2015 |