Impact of indigenous microbiota of subtidal sand on fecal indicator bacteria decay in beach systems: a microcosm study†
Qian
Zhang
,
Xia
He
and
Tao
Yan
*
Department of Civil and Environmental Engineering, University of Hawaii at Manoa, 2540 Dole Street, 383 Holmes Hall, Honolulu, HI 96822, USA. E-mail: taoyan@hawaii.edu; Fax: +1 808 956 5014; Tel: +1 808 956 6024
First published on 3rd March 2015
Abstract
Fecal contamination of coastal recreational water can adversely impact the public health and economic well-being of coastal communities. The current recreational water management practices focus primarily on water itself, while recent studies have identified other beach system components that can impact water quality. The objective of this study was to use microcosms to determine whether subtidal beach sand can enhance the decay of fecal bacteria and identify underlying mechanisms. The decay patterns of exogenous Enterococcus faecalis cells in laboratory beach microcosms for three beaches in Hawaii were determined, and beach sand indigenous microbiota was identified to be the major factor correlating to bacterial decay rates. Subsequent experiments observed that higher indigenous microbiota corresponded to faster bacterial decay. Comparison between the two major beach system components (beach sand and seawater) indicated that the indigenous microbiota in beach sand played a significant role in bacterial decay. Manipulating two important beach characteristics (sand-to-water ratio and sand particle size) that relate to indigenous microbiota abundance also resulted in different bacterial decay rates. The significant contribution of beach sand and its indigenous microbiota to fecal bacteria decay identified a positive function of beach sand in beach water quality management, which supports the inclusion of beach sand in beach quality management.
Water impactThe current recreational water management practices focus primarily on beach water itself and have traditionally neglected the impact of beach sand on water quality. Beach sand can contain a high level of fecal indicator bacteria and hence is sometimes considered an adverse factor to beach water quality. This study used laboratory microcosms to illustrate that the presence of subtidal beach sand enhanced the decay of fecal indicator bacteria, and the enhancement was caused by the indigenous sand microbiota. This finding will help develop a more comprehensive understanding of the natural processes in beach systems and improve beach water management practices. |
Introduction
Coastal beach water quality is important to the public health and economic well-being of coastal communities. Recreational water is an important transmission route of waterborne pathogens and can result in large-scale disease outbreaks;1,2 for example, in 2009–2010, U.S. CDC reported 81 recreational water-associated disease outbreaks.3 To protect the public from health risks associated with contaminated recreational water, beach advisories and closures are frequently issued. The National Research Defense Council (NRDC) reported that in 2009 beach water pollutions caused beach closures and advisories to exceed 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 for the fourth consecutive year in the U.S.4 A separate survey by the U.S. EPA indicated that 40% of 3762 beaches monitored in 2012 experienced at least one advisory or closure.5 Frequent beach advisories and closures can hurt the economy of coastal communities, which increasingly relies on tourism and recreation,4 and the impact can be very significant because about 75% of the world's unfrozen shorelines are sandy beaches.6
000 for the fourth consecutive year in the U.S.4 A separate survey by the U.S. EPA indicated that 40% of 3762 beaches monitored in 2012 experienced at least one advisory or closure.5 Frequent beach advisories and closures can hurt the economy of coastal communities, which increasingly relies on tourism and recreation,4 and the impact can be very significant because about 75% of the world's unfrozen shorelines are sandy beaches.6
The current recreational water quality regulation paradigm is primarily underpinned on beach water itself.7,8 However, recently a systems view that encompasses all beach system components, including beach sand,9,10 sediment,11,12 and aquatic vegetation,13,14 is developing. In particular, beach sand, the characteristic feature of a beach system, has recently been identified as a potential secondary reservoir of fecal indicator bacteria (FIBs). Higher levels (10- to 100-fold on a unit mass basis) of fecal indicator Escherichia coli and enterococci were frequently detected in beach sand than in the corresponding beach water.9,15–22 Given the significant sand mass in a beach system and its continuous interactions with water,17,23–25 FIBs-laden beach sand can act as significant secondary reservoirs of FIBs, which can potentially cause chronic water quality deterioration and hamper proper health risk assessment.
Although numerous studies (discussed above) have examined the negative impact that contaminated beach sand can exert (i.e., as chronic sources of FIBs), few studies have investigated the potential positive roles that beach sand may play in beach water quality protection. This is important as various natural phenomena and anthropogenic activities can significantly change the quantity and quality of beach sand in beach systems. Beach erosion, which gradually leads to considerable sand loss, is widespread at present and expected to worsen in the future as a result of global warming.26 Beach nourishment programs, responding to beach erosion, can abruptly change the sand dynamics, affecting both sand quantity and quality. In response to chronic water quality issues implicating contaminated beach sand, sand replacement has become a viable remediation option,27 while some agencies even advocate removing beach sand as a potential solution. Therefore, a full understanding of the various impact (positive and negative) that beach sand can have on beach water quality is essential to the development of a comprehensive, systems approach in beach management.
Beach sand can be expected to positively impact beach water biological quality via various physical processes that can remove fecal bacteria from water, including adsorption, straining and absorption. Although no studies in the literature have directly characterized how these physical processes remove fecal bacteria from beach water, these processes have been extensively studied in sand filtration for water treatment, and hence similar functions can be expected of beach sand in principal. Less understood is how beach sand contributes to the decay of fecal bacteria that are either adsorbed onto the sand particle surfaces, trapped within sand cavities, absorbed into the sand biofilm, or simply present within the interstitial water.
Various abiotic and biotic environmental factors can affect the decay of fecal bacteria in natural environments. Common abiotic environmental stresses include temperature variation,28 sunlight inactivation,29,30 carbon starvation,31 pH fluctuation,32 and osmotic stress from salinity changes,33 and their effects on the decay of fecal bacteria in the environment are generally well understood. Biotic processes, which typically include protozoa predation,34,35 phage infection36,37 and bacterial antagonism,38–42 on the other hand, have received limited attention. Surprisingly, recent studies have shown that the biotic processes associated with indigenous microbiota are very important to fecal bacteria decay in the environment. For example, microcosm studies that compared the survival of fecal indicator E. coli and Enterococcus faecalis in sterile and non-sterile soil,43–45 freshwater,43,44 seawater,46 and beach sand42,47 often reported more than 10-fold faster decay rates in the presence of indigenous microbiota, and the removal of indigenous microbiota even drastically reduced the effect of abiotic stresses, such as sunlight inactivation,48 which were traditionally believed to be the major forces behind bacterial decay.
The objective of this study was to investigate whether subtidal beach sand can enhance the decay of fecal bacteria in beach systems using laboratory microcosms. Initially, the decay patterns of E. faecalis in beach microcosms containing subtidal sand and seawater samples from three natural beaches were determined, and the decay rates were compared with common physicochemical and microbiological parameters of the beach sand and seawater samples. Then, the impact of indigenous beach microbiota was verified by changing its abundance level in microcosms and comparing the corresponding bacterial decay rates. Subsequently, the relative contributions from beach sand and seawater were isolated and determined by comparing bacterial decay rates in microcosms that contained either both beach sand and seawater or seawater only. Microcosms containing autoclaved beach sand and seawater were used concurrently to quantify the matrix effects in bacterial decay. Finally, the impact of different beach characteristics, including sand particle size and sand-to-water ratio, on bacterial decay was also investigated.
Materials and methods
Beach sand, seawater, and wastewater sampling
Beach sand samples were collected from three beaches on the Island of Oahu, Hawaii, including Waialae Beach (21.26968° N; 157.77710° W), Kailua Beach (21.40222° N; 157.7394° W), and Kualoa Beach (21.51330° N; 157.8360° W). At each sampling site, four subtidal sand samples were collected at knee depth and from a 0.5 m radius of the standing position, and the four samples were pooled together and stored in sterile Whir-Pak sampling bags. Seawater samples were collected at the same sampling locations using sterilized wide-mouth plastic bottles. Grab raw municipal wastewater samples were collected from the headwork of the Sand Island Wastewater Treatment Plant (SIWTP) (Honolulu, HI) in sterilized wide-mouth plastic bottles. All samples were stored at 4 °C and in the dark during transportation to the laboratory for immediate processing and analysis.Sample characterization and analysis
The beach sand samples were characterized and analyzed to determine various physicochemical and biological parameters. Total bacterial density in the beach sand samples and seawater was conservatively estimated using heterotrophic plate count (HPC). Sand extraction followed a procedure described by Boehm et al.,49 which involved shaking 10 grams of sand in 100 mL of sterilized deionized water for 2 minutes by hand and collecting supernatants of the sand extracts after settlement for 30 seconds. Supernatants of the sand extracts and the seawater samples were subjected to tenfold dilution, spread platted on tryptic soy agar (TSA), and incubated at 37 °C for 24 hours before colony enumeration.pH and salinity of beach sand extracts and seawater samples were determined using a benchtop pH meter (UltraBasic; Denver Instrument) and an Orion 150A plus conductivity meter (Thermo, Waltham, MA), respectively. The total organic carbon (TOC) and total nitrogen (TN) of sand extracts and seawater samples were determined using a TOC analyzer coupled to a TN detector (Shimadzu, Kyoto, Japan). Biochemical oxygen demand (BOD) of sand extracts and seawater was determined using a YSI Model 58 Dissolved Oxygen Meter for a 5-day BOD test (BOD5) following the APHA 5210B standard method.50
Bacterial strain and enumeration
E. faecalis strain ATCC 29212 was used as the model organism to represent fecal bacteria in this study. The preparation of stationary-stage cell suspensions followed the procedure described previously by Feng et al.42 In brief, stationary-stage E. faecalis cells (OD600 nm ≥ 1.2) were harvested by centrifugation at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 × g for 2 minutes from cultures in tryptic soy broth (TSB) that were cultivated at 37 °C with constant shaking (200 rpm). The harvested cell pellets were washed (by resuspension and precipitation) twice with phosphate-buffered saline (PBS) (pH = 7.0) and then suspended in PBS to prepare working cell suspensions (ca. 108 CFU mL−1). For the enumeration of E. faecalis cells in the laboratory microcosm experiments, sand samples collected from the beach microcosms (described below) were extracted by following the sand extraction procedure described above. E. faecalis cells in the sand extracts and water samples were enumerated using the membrane-Enterococcus indoxyl-β-D-glucoside (mEI) agar method.51
000 × g for 2 minutes from cultures in tryptic soy broth (TSB) that were cultivated at 37 °C with constant shaking (200 rpm). The harvested cell pellets were washed (by resuspension and precipitation) twice with phosphate-buffered saline (PBS) (pH = 7.0) and then suspended in PBS to prepare working cell suspensions (ca. 108 CFU mL−1). For the enumeration of E. faecalis cells in the laboratory microcosm experiments, sand samples collected from the beach microcosms (described below) were extracted by following the sand extraction procedure described above. E. faecalis cells in the sand extracts and water samples were enumerated using the membrane-Enterococcus indoxyl-β-D-glucoside (mEI) agar method.51
Beach microcosm setup
Beach microcosms were constructed in clean and autoclaved Mason jars (500 mL) and contained beach sand (100 g) and seawater (200 mL) collected from the three beaches. Stationary-stage E. faecalis cell stock solutions were freshly prepared, and 8 mL of the stock solution, which gave ca. 109 cells in total, was spiked into each beach microcosm. The microcosms were mixed using a sterile stainless steel spatula for two minutes. After thorough mixing, the microcosms were incubated in the dark at room temperature (22–24 °C) with gentle constant shaking (100 rpm), which facilitated water circulation without slurry formation. During each sampling, the sand and water compartments of the microcosms were temporarily separated by completely draining the water into sterile flasks. Water samples were collected from the completely mixed flasks, while the drained sand in the microcosms was completely mixed and sampled. After sampling, the microcosms were reconstituted, and incubation resumed until the next sampling. This sampling strategy was necessary to avoid extensive mixing that would be required if the sand and water in a microcosm were to be sampled together. All experimental treatments in this study used three replicate microcosms as biological replicates, and the same microcosm setup was used throughout this study unless stated otherwise.Manipulation of indigenous microbial communities
The indigenous microbiota of beach sand and seawater samples collected from the Waialae beach was altered either by antibiotic treatment or by autoclaving. Streptomycin was selected as the antibiotic agent based on a preliminary test, which showed that 500 mg L−1 of streptomycin reduced the culturable counts of HPC in beach microcosms by more than 10 fold within 24 hours without significantly affecting the culturable counts of E. faecalis cells (>95% culturable). Microcosms with antibiotic treatment were established as described above and then amended with streptomycin to reach a final aqueous concentration of 500 mg L−1. For the microcosms containing autoclaved beach sand and seawater, the microcosms were autoclaved at 121 °C for 20 min on two consecutive days before the amendment of E. faecalis cells aseptically.Alteration of beach sand and seawater composition in microcosms
The beach sand and seawater composition in microcosms was also altered. Beach sand and seawater samples collected from the Waialae beach were used. The first set of microcosms used two different sand-to-water mass ratios (0 and 0.5). The seawater-only microcosms (sand-to-water mass ratio = 0) contained zero gram of beach sand and 200 mL of seawater, while microcosms with a sand-to-water ratio of 0.5 contained 100 grams of beach sand and 200 mL of seawater. Control microcosms for both groups of microcosms were established using autoclaved beach sand and seawater samples. The second set of microcosms used three different sand-to-water mass ratios (0.25, 0.5 and 1.0); the microcosms contained the same volume of seawater (200 mL) and 50 g, 100 g, and 200 g of beach sand, respectively. The third set of microcosms used the same sand-to-water mass ratio (i.e., 100 g of sand and 200 mL of seawater) but differed in sand particle sizes, which resulted in different total surface sand areas in the beach microcosms. The sand samples collected from the Waialae beach were wet sieved using standard testing sieves (USA Standard Testing Sieve) to collect three different size fractions (particle diameters <1.20 mm, between 1.40 and 2.00 mm, and between 2.00 and 2.36 mm). The different total surface areas of the different size fractions were expected to provide different quantities of total biomass of indigenous microbiota, as the finer sand particles provide higher specific surface area and typically contain more organic-rich micropatches.Decay of E. coli from raw wastewater in beach microcosms
Beach sand and seawater microcosm were also spiked with 20 mL of raw wastewater collected from the SIWTP. Beach sand and seawater collected from the Waialae beach were used to construct microcosms as described above. Beach sand and seawater samples from the microcosms were processed as described above, and the concentration of E. coli in the samples was enumerated by a membrane filtration method using the modified mTEC agar according to EPA method 1603.52Data analysis
The number of culturable E. faecalis cells in beach microcosms over time was fitted into the 1st-order decay model (ln(Mt/M0) = −kdt) to determine the 1st-order decay rate kd. Because the beach sand microcosms contain two different matrices (i.e., sand and water), it was more convenient to track the total number of culturable E. faecalis cells (M) in each microcosm, which includes cells in both the beach sand and the seawater compartments. Mt and M0 are the total numbers of culturable E. faecalis cells in the microcosms at time t and time zero, respectively. The number of culturable E. faecalis cells in microcosms containing autoclaved beach sand and seawater was fitted into the same 1st-order decay model (ln(Mt/M0)a = −kdat), where kda is the decay rate of E. faecalis in the absence of indigenous microbiota. The goodness of fit (r2) and the 95% confidence interval (CI) for the model fitting were obtained and used to determine the suitability of the model. To identify the decay rate solely attributable to the indigenous microbiota, the E. faecalis decay data were modeled using the equation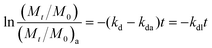 , which assumes that the overall decay rate (kd) is the summation of the decay rate in autoclaved microcosms (kda) and the decay rate contribution by the indigenous microbiota (kdl). Statistical analyses were performed using software packages SigmaPlot 12.0 and SPSS. Pearson's product moment was calculated to analyze the correlation between the decay rate of E. faecalis in beach microcosms and physicochemical and microbiological characteristics of beach sand and seawater samples. Paired t-tests were used to compare the time-series data of experimental treatments. One-way ANOVA (with Holm-Sidak post hoc test) was used to determine if a significant difference existed between different experimental treatments at given time points. The default significance level is P ≤ 0.05 unless stated otherwise.
, which assumes that the overall decay rate (kd) is the summation of the decay rate in autoclaved microcosms (kda) and the decay rate contribution by the indigenous microbiota (kdl). Statistical analyses were performed using software packages SigmaPlot 12.0 and SPSS. Pearson's product moment was calculated to analyze the correlation between the decay rate of E. faecalis in beach microcosms and physicochemical and microbiological characteristics of beach sand and seawater samples. Paired t-tests were used to compare the time-series data of experimental treatments. One-way ANOVA (with Holm-Sidak post hoc test) was used to determine if a significant difference existed between different experimental treatments at given time points. The default significance level is P ≤ 0.05 unless stated otherwise.
Results
E. faecalis decay in beach microcosms
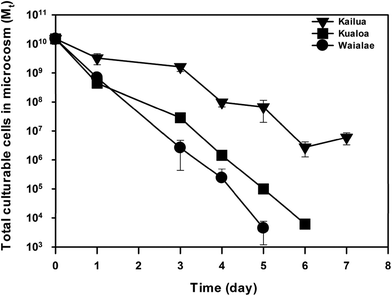
The initial physiochemical and biological parameters of the beach microcosms were determined by characterizing the beach sand and seawater samples from Waialae, Kailua, and Kualoa beaches that were used to construct the beach microcosms (Table 1). Since the indigenous enterococci counts were negligible in comparison with the exogenous E. faecalis cells amended into the microcosms (Table 1), culturable enterococci cells detected in the beach microcosms were all considered to be the exogenous E. faecalis cells. The change of culturable E. faecalis cell concentration in the sand and water compartments showed a significant difference amongst the three groups of microcosms (Fig. S1†). When the total number of culturable E. faecalis cells in each microcosm (i.e., combined from the sand and water compartments) was calculated, significantly different decay patterns were observed amongst the three groups of beach microcosms (Fig. 1). On day 1, significantly different reduction of culturable cells was observed between Kailua beach microcosms and the Kualoa and Waialae beach microcosms (ANOVA, P ≤ 0.05) (no statistical difference was observed between the Kualoa and Waialae beach microcosms). Starting from day three, a significant difference was observed amongst all three different beach microcosms (ANOVA, P ≤ 0.05), except for one comparison between the Waialae and Kualoa on day 4 (P = 0.06). Over the entire experimental course, the decay rate of E. faecalis cells in the Waialae beach microcosms (kd = 3.75 day−1; R2 = 0.92 and CI is 3.17–4.31 day−1) was larger than that in the Kualoa beach microcosms (kd = 2.90 day−1; R2 = 0.92 and CI is 2.49–3.32 day−1). The slowest decay rate was observed in the Kailua beach microcosms (kd = 1.24 day−1; R2 = 0.91 and CI is 1.05–1.43 day−1). After day 5, E. faecalis cells in the Waialae beach microcosms had decreased to below the method detection limit.| Characteristics | Beach microcosms | Pearson's r (P) | ||
|---|---|---|---|---|
| Waialae | Kailua | Kualoa | ||
| Enterococci in sand (CFU/100 mL) | 20 | 0 | 0 | — |
| Enterococci in seawater (CFU/100 g) | 16 | 7 | 0 | — |
| HPC in sand (log CFU/100 grams) | 6.39 | 4.48 | 5.66 | 1.00 (0.01) |
| HPC in seawater (log CFU/100 mL) | 5.57 | 4.45 | 5.02 | 0.99 (0.07) |
| Total HPC in microcosm (log CFU) | 6.45 | 4.76 | 5.75 | 1.00 (0.02) |
| Seawater TOC (mg L−1) | 1.36 | 1.28 | 1.27 | 0.73 (0.48) |
| Seawater TN (mg L−1) | 0.13 | 0.11 | 0.20 | 0.33 (0.78) |
| Seawater BOD5 (mg L−1) | 2.46 | 1.45 | 2.15 | 1.00 (0.06) |
| Seawater pH | 7.63 | 7.74 | 7.76 | −0.79 (0.42) |
| Seawater salinity (%) | 3.36 | 3.28 | 3.3 | 0.92 (0.26) |
| Sand TOC (mg kg−1) | 10.8 | 6.0 | 18.9 | 0.48 (0.68) |
| Sand TN (mg kg−1) | 1.70 | 0.83 | 2.5 | 0.62 (0.57) |
| Sand BOD5 (mg kg−1) | 15.8 | 10.6 | 13.4 | 1.00 (0.05) |
| Sand extraction pH | 9.08 | 9.10 | 9.07 | −0.75 (0.47) |
To identify factors contributing to the different decay rates observed in the three groups of beach microcosms, the decay rates were correlated with various initial characteristics of the beach microcosms (Table 1). Significant correlation with the decay coefficient (P ≤ 0.05) was only observed for the bacterial biomass in beach sand, the total bacterial biomass in the microcosm, and the sand BOD5. The decay coefficients also correlated with bacterial biomass in beach water (P = 0.07) and BOD5 in seawater (P = 0.06) at less significance levels. Since indigenous microbial biomass and BOD5 usually exhibit good correspondence, the observed correlations suggested a strong role of indigenous beach sand microbiota on E. faecalis cell decay in the beach microcosms.
Role of indigenous microbiota
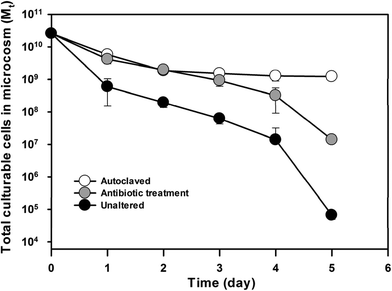
To further confirm the impact of indigenous beach microbiota on E. faecalis cell decay, beach microcosms that contain different levels of indigenous microbiota were constructed using the sand and seawater samples from the Waialae beach. The microcosm experiment included three treatments: (1) unaltered indigenous beach microbiota, (2) reduced indigenous microbial density/activity by antibiotic treatment, and (3) complete removal of indigenous microbiota by autoclaving. The indigenous microbiota strongly affected E. faecalis cell decay as indicated by the different reduction patterns of culturable cells in the sand and seawater compartments of the microcosms (Fig. S2†). The total culturable E. faecalis cells in each microcosm also exhibited different patterns as a result of the different indigenous microbiota abundance levels (Fig. 2). At the end of the experimental course (i.e. day 5), the total culturable E. faecalis cells in the three sets of microcosms showed a significant difference (ANOVA, P ≤ 0.001). Microcosms with the highest indigenous microbiota level (i.e., unaltered beach sand and seawater samples) showed the fastest decay rate (kd = 2.23 day−1; R2 = 0.91 and CI is 1.87–2.59 day−1). The beach microcosms treated with streptomycin showed significantly slower (t test, P < 0.001) E. faecalis cell decay (kd = 1.34 day−1, R2 = 0.92 and CI is 1.12–1.55 day−1) than the unaltered microcosms, while the microcosms containing autoclaved sand and seawater exhibited the smallest decay rate among the three treatments (kd = 0.58 day−1, R2 = 0.77 and CI is 0.41–0.74 day−1), which strongly indicates the important role of indigenous beach microbiota on E. faecalis cell decay in the beach microcosms.Relative contributions from beach sand and seawater
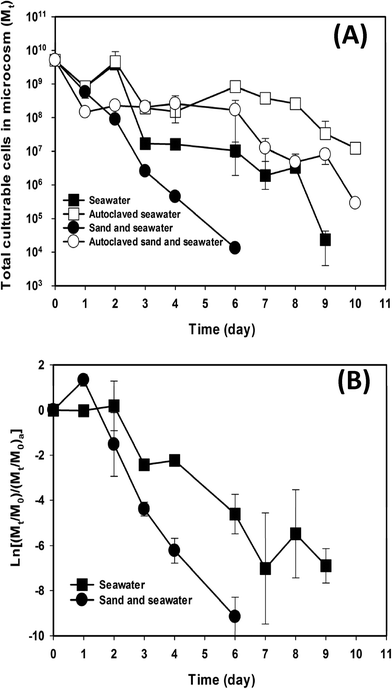
Since the contribution of indigenous microbiota on E. faecalis cell decay could be from both beach sand and seawater, relative contributions from the two beach components to the decay of exogenous E. faecalis cells were determined by comparing regular beach microcosms, which contained both beach sand and seawater, with microcosms containing only seawater. The concentration of culturable E. faecalis cells and its change over time in the microcosms are shown in Fig. S3.† The total number of culturable cells in each microcosm showed a significant difference (paired t-test: P ≤ 0.05) amongst the microcosms (Fig. 3). The beach microcosms containing both sand and seawater exhibited significantly faster (t test, P < 0.01) E. faecalis decay (kd = 2.19 day−1; R2 = 0.98 and CI is 2.02–2.37 day−1) than the beach microcosms that lacked beach sand (kd = 1.27 day−1; R2 = 0.85 and CI is 1.05–1.49 day−1) (Fig. 3A). Since some of the observed difference in decay rates may be due to the matrix effects that can differ between sand and water, autoclaved controls were established for both types of beach microcosms. Indeed, the autoclaved beach microcosms, which contained both beach sand and water, also exhibited slightly faster decay of E. faecalis cells (kda = 0.72 day−1; R2 = 0.81 and CI is 0.59–0.86 day−1) than the autoclaved seawater-only microcosms (kda = 0.46 day−1; R2 = 0.62 and CI is 0.32–0.60 day−1), which could be attributed to different endogenous decay rates and/or recovery coefficients of the E. faecalis cells due to matrix effects.In order to remove the matrix effects, the decay of E. faecalis cells in microcosms containing intact beach sand and/or seawater was divided by the decay of E. faecalis cells in the microcosms containing autoclaved beach sand and/or seawater. The resulting decay patterns still exhibited significantly faster rates (t test, P = 0.001) in the beach microcosms containing both sand and seawater than in the microcosms containing only seawater (Fig. 3B). The corresponding decay rates kdl, which quantify bacterial decay contributed by the indigenous microbiota, were 1.78 day−1 (R2 = 0.90 and CI is 1.47–2.09 day−1) and 0.87 day−1 (R2 = 0.81 and CI is 0.70–1.05 day−1) for the microcosms containing both sand and seawater and the microcosms containing seawater only, respectively. The difference in decay rates kdl clearly indicates that the indigenous beach sand microbiota contributed significantly to E. faecalis cell decay in the beach microcosms.
Impact of beach characteristics
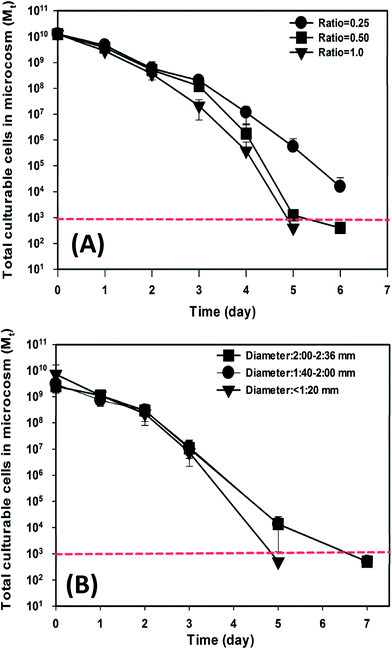
The impact of two important beach characteristics, sand-to-water ratio and sand particle size distribution, on the decay of exogenous E. faecalis cells was also investigated in beach microcosms. The concentration of culturable E. faecalis cells in the sand and water compartments showed different reduction patterns over time in respect to different sand-to-water ratios (Fig. S4†). The total number of culturable cells in each microcosm also exhibited different bacterial decay patterns (Fig. 4A). On day 5, the total number of culturable cells in the different microcosms showed a significant difference (ANOVA, P < 0.05). The beach microcosms containing the smallest amount of beach sand (i.e. sand-to-water ratio = 0.25 g mL−1) showed the slowest cell decay (kd = 2.20 day−1; R2 = 0.95 and CI is 1.96–2.48 day−1), while the microcosms containing higher ratios of sand exhibited significantly faster cell decay. The beach microcosms with a sand-to-water ratio of 0.50 g mL−1 exhibited a kd value of 2.37 day−1 (R2 = 0.89 and CI is 1.86–2.88 day−1), and the beach microcosms with a sand-to-water ratio of 1.00 g mL−1 exhibited a kd value of 2.49 day−1 (R2 = 0.95 and CI is 2.15–2.84 day−1).When different size fractions of the Waialae beach sand (particle sizes <1.2 mm, 1.4–2.0 mm, and 2.0–2.4 mm) were used to establish three experimental treatments that had the same total mass but different total surface areas (>893 cm2, 536–765 cm2, 450–536 cm2), significantly different decay patterns were also observed (Fig. S5†). The total number of culturable E. faecalis cells in each microcosm also showed clearly different reduction patterns (Fig. 4B). The beach microcosms containing the finest sand fraction (i.e. particle size <1.2 mm) exhibited a significantly faster decay rate (kd = 2.05 day−1; R2 = 0.88 and CI is 1.52–2.58 day−1) than the two sets of microcosms containing larger sand size fractions (i.e. particle size: 1.4–2.0 mm and 2.0–2.4 mm). Beach microcosms containing the two larger size fractions did not exhibit statistically distinct decay patterns (kd = 2.53 day−1; R2 = 0.93 and CI is 2.12–2.93 day−1), which was likely due to their close total sand surface areas.
Decay of wastewater E. coli in beach microcosms
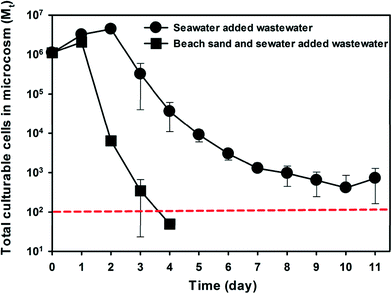
To investigate if indigenous microbiota of subtidal sand also contributes to the decay of the other common FIB E. coli, raw municipal wastewater was introduced to beach microcosms and the concentration dynamics of wastewater E. coli was monitored (Fig. 5). The microcosms containing both beach sand and seawater showed a significantly faster E. coli decay than the microcosms containing only seawater. The decay rate of E. coli in the beach sand and seawater microcosms (kd = 3.68 day−1) was 3.9 times higher than that in seawater microcosms (kd = 0.95 day−1).Discussion
The impact of beach sand and its indigenous microbiota on the decay of fecal bacteria was investigated using laboratory beach microcosms and a model indicator organism E. faecalis. Since the bacterial decay was quantified by enumerating culturable E. faecalis cells recovered from beach microcosms that contained both beach sand and seawater, the presence of two different types of matrices could result in different cell recovery efficiencies, which needs to be carefully considered in data interpretation. The use of 1st-order decay kinetic model in calculating the decay rate based on the ratio Mt over M0 partially alleviated the potential bias associated with the matrices, assuming the same recovery efficiency for the same matrix. This treatment however introduced an additional assumption, which is that bacterial cell recovery efficiency does not change over time (i.e., lack of a temporal component). The spiked E. faecalis cells can adsorb to the sand surfaces,53 which usually occurs either instantaneously or within time frames significantly shorter than the duration of this study54 and therefore were not expected to contribute a significant temporal component to the cell recovery efficiency. The E. faecalis cells may also gradually absorb into and integrate with sand surface biofilms, which could decrease cell recovery efficiency over time. Although few studies have examined this effect directly, sand extraction procedures have often reported good bacterial recovery sufficient for enumeration49 and transport studies often observed quick mobilization of enterococci in beach sand,9,55 suggesting limited temporal component of the recovery efficiency. Nevertheless, the potential temporal component of cell recovery efficiency needs to be considered during the interpretation of bacterial decay data.The first microcosm experiment showed distinct decay patterns of exogenous E. faecalis cells in microcosms containing beach sand and seawater samples from three natural beaches (Fig. 1). Correlation analysis between the bacterial decay rates and various beach sand and seawater sample characteristics only detected significant correlations with parameters related to the indigenous microbiota in beach sand, namely HPC and BOD5 (Table 1), with higher abundances of indigenous microbiota corresponding to faster bacterial decay rates. HPC is a very conservative estimator of microbial biomass because majority of microorganisms cannot be enumerated by cultivation-based methods, such as HPC. Furthermore, the use of DI water in sand extraction, which was designed specifically for recovering enterococci,49 applies hypotonic osmotic pressure to the indigenous marine microorganisms and may cause cell lysis. These limitations, however, should not hamper cross sample comparison, assuming all samples experienced the same level of biases. Since beach systems can differ at numerous aspects, the observed exclusive correlation between the decay rate of exogenous E. faecalis cells and the abundance of beach sand indigenous microbiota provided strong evidence (albeit indirect) for the study hypothesis that indigenous sand microbiota can enhance the decay of exogenous fecal bacteria. However, it should be noted that the experimental design of this particular experiment could not quantify or qualitatively exclude the contribution of varying cell recovery efficiency, especially if it had decreased over time.
To further determine the contribution of indigenous microbiota on bacterial decay and to identify the impact of cell recovery efficiency, beach microcosms that contained different levels of indigenous microbiota from the Waialae beach (unaltered, antibiotic-treated, and autoclaved) were established. A positive correspondence between the abundance of indigenous microbiota and bacterial decay rate was observed (Fig. 2), which strongly indicated a causal relationship between the two parameters. Since microcosms in this experiment were constructed of the same materials, it was possible to deduce from the bacterial decay pattern in the autoclaved treatment that the decrease in cell recovery efficiency over time in the beach microcosms was fairly limited, and hence the observed cell decay in the microcosms was primarily caused by the presence of indigenous microbiota.
Given that beach sand typically contains significant indigenous microbiota (many studies reported more than 10-fold higher bacterial biomass in beach sand than in seawater on a unit mass basis56,57), beach sand can be expected to contribute significantly to the removal of fecal bacteria in beach systems. This was clearly demonstrated in the microcosm experiment by comparing regular beach microcosms (containing both beach sand and seawater) and seawater-only microcosms, where the former exhibited significantly faster bacterial decay (kd = 2.19 day−1) than the latter (kd = 1.27 day−1) (Fig. 3A). The inclusion of autoclaved regular beach microcosms and seawater-only microcosms in this experiment further supported the observations made in Fig. 1 and 2 regarding the importance of indigenous microbiota to bacterial decay. More importantly, the decay data in the autoclaved microcosms allowed for the estimation of the temporal component of cell recovery in the different beach microcosms. The significantly faster bacterial decay rate in the autoclaved regular beach microcosms than in the autoclaved seawater-only microcosms indicated the presence of a temporal component in cell recovery efficiency (i.e., decreasing cell recovery from beach sand over time). However, even when this temporal component of cell recovery efficiency was removed by subtracting the live microcosms with the autoclaved control microcosms, a significantly faster decay rate was still observed in the microcosms containing beach sand (Fig. 3B), clearly indicating the contribution of indigenous microbiota in beach sand to the decay of exogenous E. faecalis cells.
Since various beach sand characteristics can affect the abundance of indigenous microbiota, changes in such characteristics can be expected to enhance the decay of fecal bacteria. Two beach sand characteristics, sand-to-water ratio and sand particle size distribution, which are parameters controllable during beach restoration and sand nourishment efforts, were further investigated. Higher sand-to-water ratios resulted in faster E. faecalis decay (Fig. 4A), which is in agreement with the higher decay rate observed in regular beach microcosms than in seawater-only microcosms (Fig. 2). The sand/water ratios used in this study (0.25 to 1.0) are relative high for experimental purposes and may only approximate actual sand/water ratios commonly found in the shallow portion of a beach system (e.g. knee to thigh water depth). The intensive interaction between sand and seawater in the shallow portion of beach systems deserves particular attention in recreational water management, as these areas usually see majority of recreational activities yet higher FIB concentrations are often observed in these shallow areas than in the deeper portion of beach systems. Similarly, beach microcosms containing the smallest size fraction (<1.20 mm) of the Waialae beach sand, which were expected to contain more indigenous microbiota due to the largest total surface area and the tendency of indigenous microbiota to accumulate on finer particles, exhibited faster E. faecalis decay than the beach microcosms containing larger size fractions (Fig. 4B). The two sets of beach microcosms containing larger size fractions (1.40–2.00 mm and 2.00–2.36 mm) exhibited very similar E. faecalis decay rates, which was probably due to their similar total sand surface areas and hence total abundances of indigenous microbiota.
Indigenous microbiota of natural environments represents a major factor in the decay of fecal bacteria in the environments. Many previous studies have focused on the effects of various abiotic environmental factors on the decay of fecal bacteria in aquatic environments,28–31,58,59 while other studies have suggested that the indigenous microbiota may play a significantly more important role than abiotic stresses.42–47 If the contributions of abiotic stresses and indigenous microbiota were independent and superimposable, the different decay rates would theoretically suggest a dominant role of indigenous microbiota over abiotic stresses (such as temperature fluctuation, sunlight inactivation, osmotic stress, etc.). In reality, it is more likely that indigenous microbiota interacts with abiotic stresses, which enhances each other's effects on the decay of fecal bacteria. Although the complex ecological interactions between indigenous microbiota, other environmental stresses, and exogenous fecal bacteria remain poorly characterized, which was not the main objective of this study, it should be clear that indigenous microbiota plays an important role in the decay of fecal bacteria in the environment.
Conclusions
The significant contribution of beach sand and its indigenous microbiota to the decay of E. faecalis in the beach microcosms indicates that beach sand, as the characteristic component of a beach system, not only provides recreational values to bathers but also contributes significantly to beach water quality. The contribution from beach sand goes beyond the traditional perception of contaminant adsorption and retention and involves actively facilitating the removal of fecal bacteria. This newly recognized function of beach sand should be of particular relevance to recreational water management during the early stages of fecal pollutions when large numbers of fecal bacteria are discharged into beach systems. Since other recent studies have also reported beach sand harboring a considerable amount of FIBs persistently, which are more likely to occur towards the end of contamination events, the impact of beach sand on water quality is not only substantial but may also have contrasting effects under different circumstances. Therefore, it appears appropriate for beach managers to take a systems approach that recognize beach sand as a critical component of beach water quality management.Acknowledgements
This material is based on the work supported by the Kualoa Supplemental Environmental Project Fund from the Hawaii Department of Health to T.Y. (11-093).References
- L. M. Alexander, A. Heaven, A. Tennant and R. Morris, J. Epidemiol. Community Health, 1992, 46, 340–344 CrossRef CAS.
- A. M. Abdelzaher, M. E. Wright, C. Ortega, A. R. Hasan, T. Shibata, H. M. Solo-Gabriele, J. Kish, K. Withum, G. He, S. M. Elmir, J. A. Bonilla, T. D. Bonilla, C. J. Palmer, T. M. Scott, J. Lukasik, V. J. Harwood, S. McQuaig, C. D. Sinigalliano, M. L. Gidley, D. Wanless, L. R. Plano, A. C. Garza, X. Zhu, J. R. Stewart, J. W. Dickerson, H. Yampara-Iquise, C. Carson, J. M. Fleisher and L. E. Fleming, J. Water Health, 2011, 9, 443–457 CrossRef PubMed.
- M. C. Hlavsa, V. A. Roberts, A. M. Kahler, E. D. Hilborn, T. J. Wade, L. C. Backer and J. S. Yoder, Morb. Mortal. Wkly Rep., 2014, 63, 6–10 Search PubMed.
- NRDC, Natural Resources Defense Council, https://www.nrdc.org/water/oceans/ttw/ttw2009.pdf, 2009.
- USEPA, EPA's BEACH Report: 2012 Swimming Season. EPA 820-F-13-014, http://water.epa.gov/type/oceb/beaches/upload/national_facsheet_2012.pdf Search PubMed.
- A. C. Brown and A. Mclachlan, Environ. Conserv., 2002, 29, 62–77 CrossRef.
- V. J. Cabelli, A. P. Dufour, M. A. Levin, L. J. McCabe and P. W. Haberman, Am. J. Public Health, 1979, 69, 690–696 CrossRef CAS PubMed.
- V. J. Cabelli, A. P. Dufour, L. J. McCabe and M. A. Levin, Am. J. Epidemiol., 1982, 115, 606–616 CAS.
- K. M. Yamahara, B. A. Layton, A. E. Santoro and A. B. Boehm, Environ. Sci. Technol., 2007, 41, 4515–4521 CrossRef CAS.
- R. L. Whitman, D. A. Shively, H. Pawlik, M. B. Nevers and M. N. Byappanahalli, Appl. Environ. Microbiol., 2003, 69, 4714–4719 CrossRef CAS.
- B. D. Badgley, F. I. Thomas and V. J. Harwood, Environ. Microbiol., 2011, 13, 932–942 CrossRef CAS PubMed.
- K. L. Anderson, J. E. Whitlock and V. J. Harwood, Appl. Environ. Microbiol., 2005, 71, 3041–3048 CrossRef CAS PubMed.
- M. N. Byappanahalli, D. A. Shively, M. B. Nevers, M. J. Sadowsky and R. L. Whitman, FEMS Microbiol. Ecol., 2003, 46, 203–211 CrossRef CAS.
- B. D. Badgley, B. S. Nayak and V. J. Harwood, Water Res., 2010, 44, 5857–5866 CrossRef CAS PubMed.
- S. Ishii, D. L. Hansen, R. E. Hicks and M. J. Sadowsky, Environ. Sci. Technol., 2007, 41, 2203–2209 CrossRef CAS.
- L. J. Beversdorf, S. M. Bornstein-Forst and S. L. McLellan, J. Appl. Microbiol., 2007, 102, 1372–1381 CrossRef CAS PubMed.
- K. M. Yamahara, B. A. Layton, A. E. Santoro and A. B. Boehm, Environ. Sci. Technol., 2007, 41, 4515–4521 CrossRef CAS.
- T. Kon, S. C. Weir, E. T. Howell, H. Lee and J. T. Trevors, Appl. Environ. Microbiol., 2007, 73, 1961–1967 CrossRef CAS PubMed.
- T. D. Bonilla, K. Nowosielski, M. Cuvelier, A. Hartz, M. Green, N. Esiobu, D. S. McCorquodale, J. M. Fleisher and A. Rogerson, Mar. Pollut. Bull., 2007, 54, 1472–1482 CrossRef CAS PubMed.
- T. A. Edge and S. Hill, Water Res., 2007, 41, 3585–3594 CrossRef CAS PubMed.
- R. Oshiro and R. Fujioka, Water Sci. Technol., 1995, 31, 251–254 CrossRef.
- T. Yan, F. Feng and D. Goto, Concentration Dynamics of Fecal Indicators in Hawaii's Coastal and Inland Sand, Soil, and Water During Rainfall Events, University of Hawaii at Manoa, Alexandria, VA, 2011 Search PubMed.
- E. Wheeler-Alm, J. Burke and A. Spain, Water Res., 2003, 37, 3978–3982 CrossRef CAS.
- R. L. Whitman and M. B. Nevers, Appl. Environ. Microbiol., 2003, 69, 5555–5562 CrossRef CAS.
- M. B. Nevers and R. L. Whitman, Environ. Sci. Technol., 2008, 42, 4454–4460 CrossRef CAS.
- K. Zhang, B. C. Douglas and S. P. Leatherman, Clim. Change, 2004, 64, 41–58 CrossRef.
- R. J. Hernandez, Y. Hernandez, N. H. Jimenez, A. M. Piggot, J. S. Klaus, Z. Feng, A. Reniers and H. M. Solo-Gabriele, Water Res., 2014, 48, 579–591 CrossRef CAS PubMed.
- G. J. Vasconcelos and R. G. Swartz, Appl. Environ. Microbiol., 1976, 31, 913–920 CAS.
- R. S. Fujioka, H. H. Hashimoto, E. B. Siwak and R. H. Young, Appl. Environ. Microbiol., 1981, 41, 690–696 CAS.
- L. W. Sinton, R. K. Finlay and P. A. Lynch, Appl. Environ. Microbiol., 1999, 65, 3605–3613 CAS.
- S. I. Terzieva and G. A. McFeters, Can. J. Microbiol., 1991, 37, 785–790 CrossRef CAS.
- A. F. Carlucci and D. Pramer, Appl. Microbiol., 1960, 8, 247–250 CAS.
- I. C. Anderson, M. Rhodes and H. Kator, Appl. Environ. Microbiol., 1979, 38, 1147–1152 CAS.
- R. M. Enzinger and R. C. Cooper, Appl. Environ. Microbiol., 1976, 31, 758–763 CAS.
- J. M. Gonzalez, J. Iriberri, L. Egea and I. Barcina, Appl. Environ. Microbiol., 1992, 58, 998–1004 CAS.
- K. E. Ashelford, M. J. Day and J. C. Fry, Appl. Environ. Microbiol., 2003, 69, 285–289 CrossRef CAS.
- Y. Rozen and S. Belkin, FEMS Microbiol. Rev., 2001, 25, 513–529 CrossRef CAS PubMed.
- R. Mitchell, S. Yankofsky and H. W. Jannasch, Nature, 1967, 215, 891–893 CrossRef CAS.
- R. Mitchell and Z. Nevo, Nature, 1965, 205, 1007–1008 CrossRef.
- H. W. Jannasch, Appl. Microbiol., 1968, 16, 1616–1618 CAS.
- E. Hibbing Michael, C. Fuqua, R. Parsek Matthew and S. B. Peterson, Nat. Rev. Microbiol., 2010, 8, 15–25 CrossRef CAS PubMed.
- F. Feng, D. Goto and T. Yan, FEMS Microbiol. Ecol., 2010, 74, 214–225 CrossRef CAS PubMed.
- G. Bogosian, L. E. Sammons, P. J. Morris, J. P. O'Neil, M. A. Heitkamp and D. B. Weber, Appl. Environ. Microbiol., 1996, 62, 4114–4120 CAS.
- G. J. Medema, M. Bahar and F. M. Schets, Water Sci. Technol., 1997, 35, 249–252 CrossRef CAS.
- R. E. Andrews Jr., W. S. Johnson, A. R. Guard and J. D. Marvin, Can. J. Microbiol., 2004, 50, 957–966 CrossRef PubMed.
- A. F. Carlucci, P. V. Scarpino and D. Pramer, Appl. Microbiol., 1961, 9, 400–404 CAS.
- A. Hartz, M. Cuvelier, K. Nowosielski, T. D. Bonilla, M. Green, N. Esiobu, D. S. McCorquodale and A. Rogerson, J. Environ. Qual., 2008, 37, 898–905 CrossRef CAS PubMed.
- A. Korajkic, P. Wanjugi and V. J. Harwood, Appl. Environ. Microbiol., 2013, 79, 5329–5337 CrossRef CAS PubMed.
- A. B. Boehm, J. Griffith, C. McGee, T. A. Edge, H. M. Solo-Gabriele, R. Whitman, Y. Cao, M. Getrich, J. A. Jay, D. Ferguson, K. D. Goodwin, C. M. Lee, M. Madison and S. B. Weisberg, J. Appl. Microbiol., 2009, 107, 1740–1750 CrossRef CAS PubMed.
- American Public Health and Association, 2012.
- USEPA, EPA 821-R-02-022, United States Environmental Protection Agency, Office of Water, Washington, DC, 2002.
- USEPA, EPA 821-R-02-022, United States Environmental Protection Agency, Office of Water, Washington, DC, 2009.
- A. L. Mills, J. S. Herman, G. M. Hornberger and T. H. Dejesus, Appl. Environ. Microbiol., 1994, 60, 3300–3306 CAS.
- G. Chen, M. Rockhold and K. A. Strevett, Res. Microbiol., 2003, 154, 175–181 CrossRef.
- T. L. Russell, K. M. Yamahara and A. B. Boehm, Environ. Sci. Technol., 2012, 46, 5988–5996 CrossRef CAS PubMed.
- H. Cui, K. Yang, E. Pagaling and T. Yan, Appl. Environ. Microbiol., 2013, 79, 3601–3609 CrossRef CAS PubMed.
- H. M. Khiyama and J. C. Makemson, Appl. Microbiol., 1973, 26, 293–297 CAS.
- A. F. Carlucci and D. Pramer, Appl. Microbiol., 1960, 8, 243–247 CAS.
- S. A. Anderson, S. J. Turner and G. D. Lewis, Water Sci. Technol., 1997, 35, 325–331 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ew00004a |
| This journal is © The Royal Society of Chemistry 2015 |