Open Access Article
Open Access ArticleEvaluating the PAS-SIM model using a passive air sampler calibration study for pesticides†
Andrés Ramírez
Restrepo
ab,
Stephen J.
Hayward
ac,
James M.
Armitage
*a and
Frank
Wania
a
aDepartment of Physical and Environmental Sciences, University of Toronto Scarborough, 1265 Military Trail, Toronto, Ontario M1C 1A4, Canada. E-mail: james.armitage@utoronto.ca; Tel: +1 416-287-7225
bGrupo GDCON, Facultad de Ingeniería, Sede de Investigaciones Universitarias (SIU), Universidad de Antioquia, Calle 70 No 52 -21, Medellín, Colombia
cDepartment of Chemical Engineering and Applied Chemistry, University of Toronto, 200 College St W, Toronto, Ontario M5S 3E5, Canada
First published on 9th June 2015
Abstract
The main objective of this study was to evaluate the performance of a model for simulating the uptake of various pesticides on passive air samplers (PAS). From 2006–2007 a series of PAS using XAD-resin were deployed at Egbert, a rural agricultural site in southern Ontario, Canada, to measure the uptake of pesticides for time periods ranging from two months to one year. A continuous increase in sequestered amounts was observed for most pesticides, except for trifluralin and pendimethalin, which could conceivably be subject to substantial degradation inside the sampler. Continuous low-volume active air samples taken during the same period, along with data on weather conditions, allowed for the simulation of the uptake of the pesticides using the model (PAS-SIM). The modelled accumulation of pesticides on the PAS over the deployment period was in good agreement with the experimental data in most cases (i.e., within a factor of two) providing insight into the uptake kinetics of this type of sampler in the field. Passive sampling rates (PSR, m3 d−1) were determined from the empirical data generated for this study using three different methods and compared with the PSRs generated by the model. Overall, the PAS-SIM model, which is capable of accounting for the influence of temperature and wind variations on PSRs, provided reasonable results that range between the three empirical approaches employed and well-established literature values. Further evaluation and application of the PAS-SIM model to explore the potential spatial and temporal variability in PAS uptake kinetics is warranted, particularly for established monitoring sites where detailed meteorological data are more likely to be available.
Environmental impactPassive air samplers are frequently deployed in the field in order to monitor ambient concentrations of various contaminants in the atmosphere. Although the basic principles underlying the accumulation of organic chemicals on passive air samplers are well-established, interpretation of monitoring data is complicated by varying ambient concentrations and meteorological conditions over time. This study reports on the performance of a modeling tool (PAS-SIM) for simulating the accumulation of organic chemicals on XAD-2 passive air samplers using a calibration study for pesticides. The modelled accumulation of pesticides on the PAS was in good agreement with the experimental data in most cases (i.e., within a factor of two) providing insight into the uptake kinetics of this type of sampler in the field. |
Introduction
Pesticides have long been identified as chemicals posing a potential threat to the environment. They are often detected in regions remote from their original use, reflecting relatively high application rates and sufficient long-range transport potential (LRTP).1–6 Because the atmosphere plays a significant role in pesticide transport and fate, numerous studies report the levels of pesticides in ambient air.2–5,7 Although many sampling methods are available, passive air samplers (PAS) are often chosen for deployments over long periods at multiple sites, due to their low cost and maintenance requirements. To calculate volumetric air concentrations from the amount of a chemical quantified in a PAS, it is necessary to employ an estimated passive sampling rate (PSR). PSRs are often calculated as part of a calibration study, using independently derived air concentrations from active air samplers (AAS). Most calibrations rely on intermittent active sampling although recently continuous active samplers have been used, but only for relatively short periods.8–12 Previous work suggests that sampling rates of PAS can vary with climate and between compounds and different approaches have been proposed to account for these effects.13–15 To date however, calibrations yielding PAS sampling rates for pesticides are limited, especially in temperate regions and over extended deployment periods.11Recently, the PAS-SIM model was presented as a tool for simulating the behaviour of organic chemicals on PAS using divinylbenzene-styrene-co-polymeric resin (XAD-2) as sorbent under different exposure scenarios.16,17 One potential use of the PAS-SIM model is to estimate PSRs prior to actual deployments, based only on the meteorological conditions (i.e., temperature, wind speed) at the sampling sites and the chemical properties of the target analytes. The model's performance has been evaluated for PCBs and PAHs but not for pesticides. Accordingly, the main objective of this study is to assess the performance of the PAS-SIM model for simulating the uptake kinetics of various pesticides on PAS. PAS using XAD-2 as sorbent (hereinafter referred to as XAD-PAS) were deployed for up to one year, alongside a continuous active air sampler. The active air sampling data in combination with the PAS-SIM model were used to simulate the uptake of pesticides in the XAD-PAS. Sampling rates for a range of pesticides were derived by using the PAS-SIM model and compared with those obtained by direct data calibration methods. Three empirical methods for estimating PSRs were considered. A secondary objective of this study is therefore to provide guidance on the appropriateness and applicability of these methods for deriving empirical sampling rates from calibration studies using XAD-PAS.
Methods
Field calibration study
From March 2006 to February 2007, ambient air was sampled at the Center for Atmospheric Research Experiments (CARE), in Egbert, Ontario, Canada (44°13′52′′N, 79°46′59′′W) using a low-volume active air sampler (LV-AAS) and a set of 10 XAD-PAS. The LV-AAS operated continuously for consecutive 14-day periods, while all XAD-PAS were set out in March 2006 and then retrieved in pairs, in approximately 2-month intervals (4-month interval for the final pair retrieved in February 2007). Accordingly, there are five XAD PAS deployment lengths, (i) two months (March 1–April 27, 2006), (ii) four months (March 1–June 30, 2006), (iii) six months (March 1–September 1, 2006), (iv) eight months (March 1–October 27, 2006) and (iv) twelve months (March 1, 2006–February 27, 2007). The LV-AAS sampling has been detailed in previous papers.5,11 Briefly, a BGI-400S LV-AAS (BGI Inc.) was used to aspirate air through a PUF-XAD-PUF sandwich (5 g of XAD, between 2 cm × 3 cm polyurethane foam, PUF). The pump was calibrated to sample 2.9 ± 0.2 m3 d−1 resulting in a sample volume of 40.6 m3 for each two-week sample. The sampler was not equipped with a glass fibre filter (GFF) and hence no distinction between gaseous and particle-bound fractions can be made. The XAD-PAS, based on the design described by Wania et al.,16 consists of a stainless steel mesh cylinder (10 cm long) containing pre-cleaned XAD-2 resin, which is protected from precipitation by a stainless steel housing designed to minimize the effect of wind speed on the sampling rate.Chemical analysis
The list of chemicals measured is provided in the ESI, Section S1† and includes both Current Use Pesticides (CUPs) and Historic Use Pesticides (HUPs). The majority of the LV-AAS data were reported in a 2010 publication5 whereas only the data for the XAD-PAS pair deployed for 12 months have been reported previously.11 LV-AAS and XAD-PAS samples were extracted and analyzed using the same basic methods.5,7,11,16 Briefly, the samples were spiked with d14-trifluralin and d10-chlorpyrifos to assess method recovery and Soxhlet-extracted using dichloromethane for 24 hours. Extracts were reduced in volume, solvent exchanged into iso-octane, and analyzed by gas chromatography with a mass-selective detector in either negative chemical ionization (NCI) or electron ionization (EI) mode. Additional details of the analytical methods are reported in Section S1 in the ESI.†Empirical sampling rate derivation
Passive sampling rates were obtained from the field calibration data using three different methods. Method 1 uses the following equation: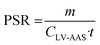 | (1) |
| m = PSR(CLV-AAS·t) | (2) |
PSR is then derived as the slope of the linear regression of the sequestered amount m against the product of CLV-AAS and time (Method 2).
Methods 1 and 2 assume the PSR to be constant during deployment, but previous research demonstrated that PSRs can vary with temperature and wind speed.7,13–15,18 To address this concern, a third method was used to account for seasonal variations. Sampling rates during each two months interval between retrievals were derived from the increase in the amount captured by subsequently retrieved XAD-PAS and the average air concentration (CLV-AAS)i, during the interval (Method 3).
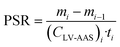 | (3) |
The use of depuration compounds has also been proposed as a method to estimate PSRs from PAS data (in the absence of AAS), based on the loss of the spiked compound over the deployment period.9,19,20 This approach is predicated on the assumption that uptake of target analytes and loss of the depuration compounds are subject to the same transport resistances, where typically it is assumed that transport across the air-side boundary layer is rate limiting. As discussed previously, transport through the porous medium on the sampler-side is an important consideration and hence these assumptions may not be valid.21,22 Additional research is required to better understand the use of depuration compounds for estimating PSRs as a function of chemical properties and environmental conditions. Regardless, because the XAD-PAS deployed in this study were not spiked with depuration compounds, this method cannot be applied to the current data.
PAS-SIM model application and evaluation
Uptake of pesticides on XAD-PAS was simulated by the PAS-SIM model17 using physical–chemical property data and site-specific meteorological data as inputs. The following compounds were simulated: alachlor, atrazine, cis-chlordane, trans-chlordane, chlorothalonil, DCPA (dimethyl tetrachloroterephthalate, dacthal), disulfoton, endosulfan I, endosulfan II, endosulfan sulfate, hexachlorobenzene (HCB), α-hexachlorocyclohexane (α-HCH), γ-HCH, metolachlor, trans-nonachlor, pendimethalin and trifluralin. Daily weather conditions for temperature used Egbert CARE facility data whereas wind speed was retrieved from Toronto Pearson International Airport (70 km away from the sampling site). These inputs are documented in the ESI† of the original PAS-SIM model publication.17 Pesticide properties (e.g., molecular weight) were retrieved from the EURL Data Pool.23 Sampler-air (KSA) and aerosol-air (KQA) partition coefficients at 25 °C were calculated using poly-parameter linear free energy relationships (pp-LFERs) and solute descriptors.24–29 The temperature dependence of the partition coefficients was estimated using the internal energy of phase change (ΔUij, kJ mol−1) according to previously reported equations.29,30 If necessary, solute descriptors were estimated using ACD/Labs software (Absolv in ACD/ADME Suite v. 5.0.8). Model inputs, solute descriptors and partition coefficients can be found in Section S2 in the ESI.†The LV-AAS data was used as an input to the model, and the output (i.e., the amount m sequestered in the PAS) was compared against the empirical data obtained from the deployed XAD-PAS. The normalized residuals error (NRE) in the model estimation was calculated using the following equation:31
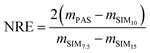 | (4) |
Results and discussion
Pesticide monitoring by XAD-PAS and LV-AAS
The sequestered amounts in each of the XAD-PAS deployed at Egbert in 2006–2007 are reported in Section S4 in the ESI,† while the recovery corrected amounts of pesticides can be found in Section S5,† and the averages in Section S6.† Seventeen pesticides (alachlor, atrazine, chlorothalonil, DCPA, disulfoton, endosulfan I, endosulfan II, endosulfan sulfate, metolachlor, pendimethalin and trifluralin, cis-chlordane, trans-chlordane, HCB, α-HCH, γ-HCH, and trans-nonachlor) were consistently detected. Some pesticides, including chlorothalonil, DCPA, endosulfan I, pendimethalin, trifluralin, HCB, α-HCH and γ-HCH, were detected even after the shortest deployment time of two months. The other pesticides may have levels below the detection limit of the analytical methods in this early spring period due the strong seasonal variability in pesticide use. Data retrieved during these two months are in agreement with the LV-AAS measurements except for pendimethalin, as it was detected in the XAD-PAS but not in any LV-AAS. When estimating an air concentration from a PAS operating in the linear uptake phase, it is assumed that only negligible amounts of the chemicals accumulated by the sampler are lost during deployment.16 The sampling strategy was designed to assess the validity of this assumption for pesticides. A non-uniform increase with larger amounts of pesticide accumulating in the PAS between June and September than during winter is consistent with higher ambient air concentrations during the growing season as has been observed by active sampling.11 Pesticides which are not in current use (HUPs such as HCB, α-HCH, γ-HCH, cis-chlordane, trans-chlordane, and trans-nonachlor), on the other hand, show continuously and uniformly rising sequestered amounts throughout the year of deployment, consistent with a lack of significant seasonal change in air concentrations related to agricultural applications. The results for these chemicals allow us to assess whether or not equilibrium between the PAS sorbent XAD and the atmosphere was approached. If we assume that these ‘historic-use’ pesticides have relatively constant concentrations in air, their net uptake in the PAS would have decreased or approached zero if they had reached equilibrium; but they did not, even though the HUPs are among those with the lowest sampler uptake capacity (quantified by the sampler-air partition coefficients KSA, whose numerical values can be found in the ESI, Section S2†) within the group of detected pesticides. Thus, we also can be confident that the other chemicals with higher sampler-air partition coefficients did not approach equilibrium during deployment either.On the other hand, whereas the LV-AAS air concentrations of pendimethalin and trifluralin remain elevated throughout the summer months (≥91 pg m−3 and 88 pg m−3 respectively), the empirical XAD-PAS data do not show continued uptake even though the amounts accumulated on the samplers (4.4 ng and 3.2 ng respectively by Sept 1) do not appear to reflect equilibrium partitioning at any time. For example, the expected amount of trifluralin on the XAD-PAS samplers deployed for this study at equilibrium with an air concentration of 20 pg m−3 at 30 °C is 13.2 ng. However, the measured amount of trifluralin on the XAD-PAS was ≤4.2 ng throughout the summer (i.e., well below the amount corresponding to thermodynamic equilibrium with the ambient air). Accordingly, the apparent loss of pendimethalin and trifluralin from the passive samplers cannot be explained by fluctuations in ambient air concentrations or enhanced volatilization in the summer caused by warmer temperatures because the sampler is below the expected equilibrium. Although both of these compounds exhibit relatively large 2nd-order OH radical reaction rate constants (kOH, as estimated using the EPISUITE AOPWIN v1.92 module), so do some of the other compounds sampled here (e.g., atrazine, disulfoton). As the experimental PAS data for these other compounds is broadly consistent with expectations, it does not seem likely that reaction with OH radicals in the pore space of the sampler alone can explain the apparent discrepancies for pendimethalin and trifluralin. Moreover, the mass fraction of the compounds in the pore air of the sampler is negligible in comparison to the sorbed fraction. An alternative explanation for these observations is that degradation of the compounds within or sorbed to the passive sampler is facilitated by the sampler medium itself. For example, it was recently reported that XAD-2 ‘artificially transformed’ chlorpyrifos to its oxygenated analogue chlorpyrifos-oxon to a substantial extent whereas PUF used in the same study did not.32 Because pendimethalin and trifluralin have a similar dinitro-aniline structure, they both may be prone to the same type of reaction process(es). Additional studies are required to explore this hypothesis experimentally; PAS-SIM model simulations incorporating this process are presented below. Because of the discrepancy identified above, care must be taken when interpreting the air concentrations of pendimethalin and trifluralin, as the estimated air concentrations using the sampler data may be inaccurate.
PAS-SIM model output and evaluation
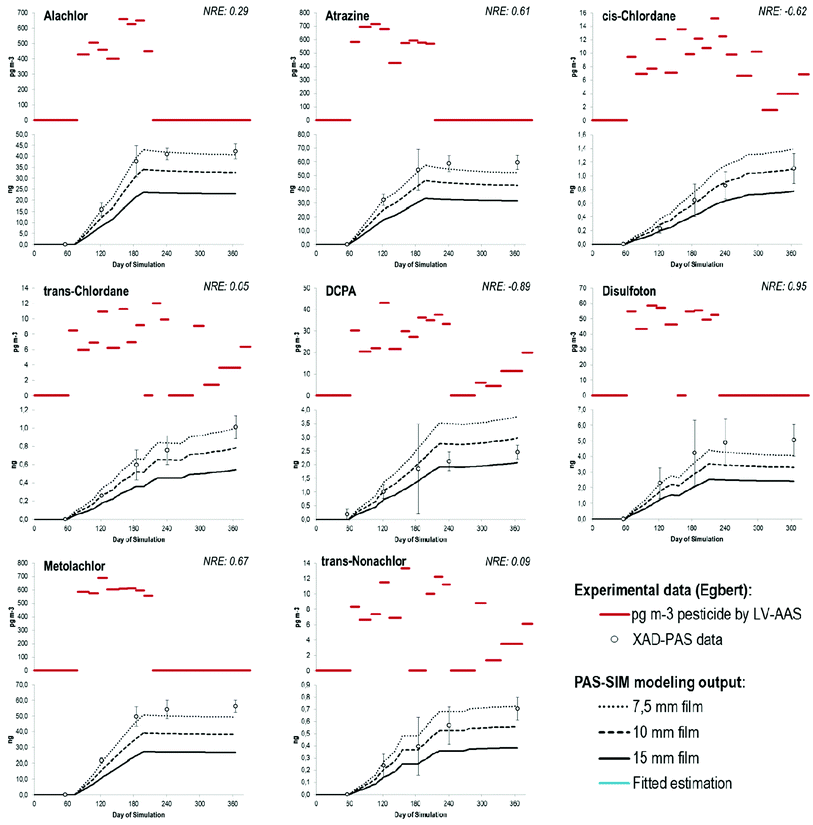
Typical results for the simulated uptake of pesticides are shown in Fig. 1–3. Concentrations of pesticides in air in Egbert measured by LV-AAS from March 1st 2006 to February 27th 2007, shown in red, were used, alongside with temperature, wind speed and chemical properties, as inputs to obtain the curves below each plot. The circles are the experimental values with their variability indicated by the error bars. The lines represent model results using different assumptions regarding the thickness of the stagnant air boundary layer: the black dotted line is the model output using a 7.5 mm thick stagnant air boundary layer; the dashed line stands for 10 mm thickness and the solid line for 15 mm thickness. As introduced above, model performance was quantified using the NRE (eqn (4)). Three levels of agreement between measurements and PAS-SIM results are defined here, (i) good agreement, (ii) systematic bias, and (iii) no agreement. Agreement was judged acceptable if the absolute NRE was below 2, i.e., if the model output was within two standard deviations (±2σ) of the experimental data. It is important to note that NRE (and FoA) results should not be interpreted as absolute criteria given that uncertainty/bias in the LV-AAS and empirical XAD-PAS data affects the performance of the model. For example, both positive and negative bias could have been introduced to the empirical XAD-PAS data through the recovery correction, which for all 17 compounds was based on only two internal standards (d14-trifluralin and d10-chlorpyrifos). Further evaluation of the PAS-SIM model by other researchers using other calibration data sets is encouraged.Of the 17 pesticides detected, acceptable agreement was found for eight compounds, seven pesticides were systematically underestimated, and there was no agreement for two pesticides. The compounds with acceptable agreement between model output and the empirical data were alachlor, atrazine, cis-chlordane, trans-chlordane, DCPA, disulfoton, metolachlor, and trans-nonachlor (Fig. 1). The emission profiles of these compounds are diverse, from pesticides with a strong seasonal variability to others with seemingly random fluctuations over time. For some pesticides in this group (DCPA, disulfoton, trans-nonachlor), not all solute descriptors had been reported in the literature so estimated values were used (i.e., Absolv output). In the case of trans-nonachlor, all the descriptors were estimated, but its NRE absolute value is less than 0.1. These results suggest that altogether the solute descriptor estimation, the pp-LFERs and the PAS-SIM model are a good and rugged assembly, able to make accurate predictions even for compounds with little experimental property data available.
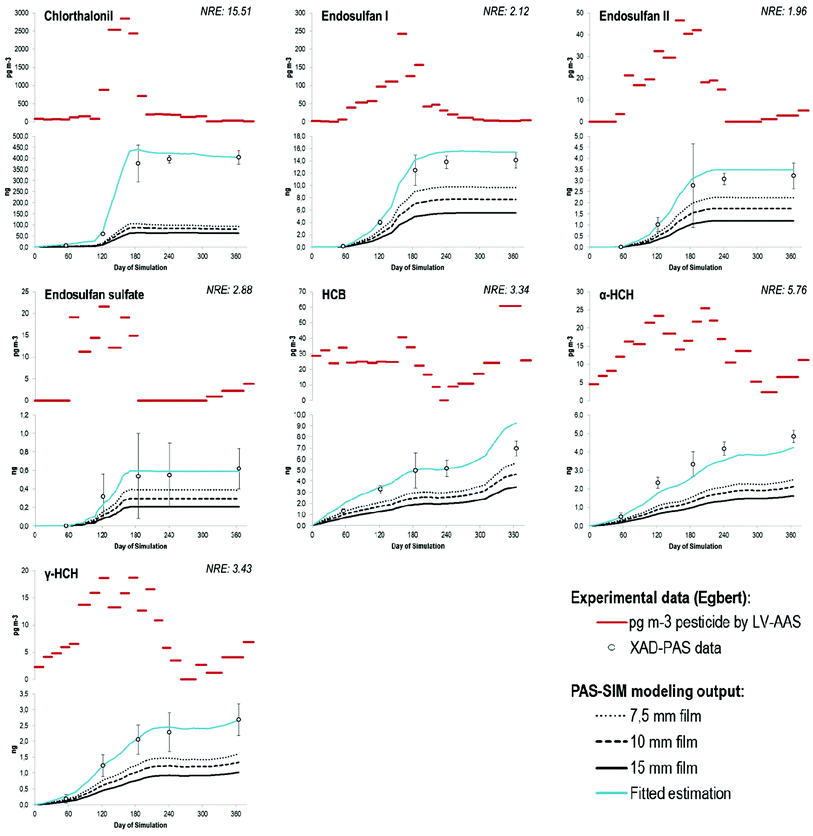
When the modeled and measured shape of the uptake curve was similar, but the NRE was systematically greater than 2σ, model results, shown in Fig. 2, were judged systematically biased. This was the case for HCB, α- and γ-HCH, the endosulfans and chlorothalonil. For the pesticides in this study, the bias was always positive, suggesting that the model is prone to underestimating the residues on XAD-PAS. The extent of bias can be expressed by the number of standard deviations n. For example, an n of approximately 3 for HCB means that the experimental values usually were three standard deviations above the predicted values. The common range for n was between 2 and 5, with the noticeable exception of chlorothalonil (n ≈ 16). Compounds with the lowest systematic bias were the endosulfans, whose structure is quite similar to the chlordanes, for which acceptable agreement was found (Fig. 1). The model bias also can be expressed by a factor of agreement (FoA), which was a factor of 2 for almost all compounds, indicating that modeled amounts (m) are approximately half of the experimental values. For chlorothalonil the modeled amounts are only a fifth of the empirical values obtained and hence the FoA is 5. The blue lines in Fig. 2 show a fitted estimation using the FoA as a correction factor for the values obtained by the model using 10 mm stagnant boundary layer. Accordingly, the values of the fitted estimates double the values obtained by the PAS-SIM model for all the compounds except for chlorothalonil, for which the fitted values are five times higher.
Uncertainty in the estimated sampler-air partition coefficients can be an important consideration for chemicals with relatively small values (i.e., log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KSA ≤ 7.5 at 25 °C). For example, model output for HCB and γ-HCH approaches the lower bound of the empirical XAD-PAS data if the log
KSA ≤ 7.5 at 25 °C). For example, model output for HCB and γ-HCH approaches the lower bound of the empirical XAD-PAS data if the log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KSAs are increased by 1 order of magnitude. The improved model performance reflects increased net uptake of the chemical (i.e., decreased volatilization) over the simulation period due to higher sorption capacity. log
KSAs are increased by 1 order of magnitude. The improved model performance reflects increased net uptake of the chemical (i.e., decreased volatilization) over the simulation period due to higher sorption capacity. log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KSA values greater than 9 did not substantially improve model performance. Although the ppLFER method used to derive all KSA values is well-validated, the potential for errors remains. As it may not be possible to know when the estimated KSAs are in fact biased low, this consideration could be taken into account as an uncertainty factor in the interpretation of PAS-SIM outputs for more volatile compounds. Bias in the estimation of the aerosol-air partition coefficient can also influence model performance if this property value is relatively large (i.e., estimated log
KSA values greater than 9 did not substantially improve model performance. Although the ppLFER method used to derive all KSA values is well-validated, the potential for errors remains. As it may not be possible to know when the estimated KSAs are in fact biased low, this consideration could be taken into account as an uncertainty factor in the interpretation of PAS-SIM outputs for more volatile compounds. Bias in the estimation of the aerosol-air partition coefficient can also influence model performance if this property value is relatively large (i.e., estimated log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KQA > 9), as the PAS-SIM model for XAD-2 assumes that the fraction of chemical bound to particulates is completely unavailable for uptake.17 Overestimation of log KQA can therefore lead to underestimation of the amount of chemical accumulated on the sampler in the model calculations because the available fraction is inaccurately quantified. This aspect may partly explain the model performance for endosulfan II and endosulfan sulfate, given that the estimated log
KQA > 9), as the PAS-SIM model for XAD-2 assumes that the fraction of chemical bound to particulates is completely unavailable for uptake.17 Overestimation of log KQA can therefore lead to underestimation of the amount of chemical accumulated on the sampler in the model calculations because the available fraction is inaccurately quantified. This aspect may partly explain the model performance for endosulfan II and endosulfan sulfate, given that the estimated log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KQAs for these compounds are greater than nine (ESI, Section S2†). For the other pesticides, this consideration is not expected to be relevant. Note that the PAS-SIM model assumptions are based on empirical XAD-PAS data for PAHs such as benzo(b)fluoranthene, benzo(a)pyrene and indeno(1,2,3-c,d)pyrene, compounds known to be predominantly particle-bound under typical atmospheric conditions. Low sampling efficiencies of particle-bound PAHs on PUF-PAS were also reported for a recent calibration study33 and the reliability of PAS for particle-bound compounds in general remains unclear.34
KQAs for these compounds are greater than nine (ESI, Section S2†). For the other pesticides, this consideration is not expected to be relevant. Note that the PAS-SIM model assumptions are based on empirical XAD-PAS data for PAHs such as benzo(b)fluoranthene, benzo(a)pyrene and indeno(1,2,3-c,d)pyrene, compounds known to be predominantly particle-bound under typical atmospheric conditions. Low sampling efficiencies of particle-bound PAHs on PUF-PAS were also reported for a recent calibration study33 and the reliability of PAS for particle-bound compounds in general remains unclear.34
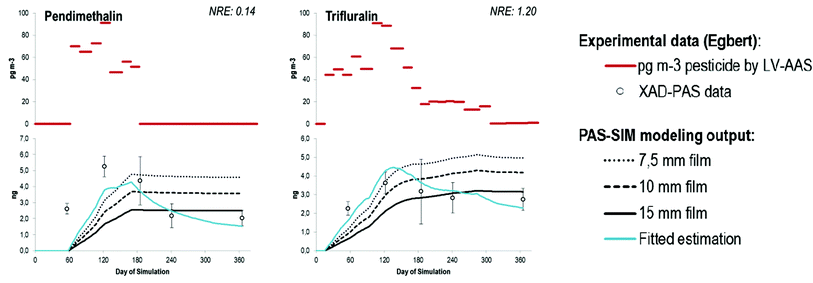
Two compounds, trifluralin and pendimethalin, showed a completely different behavior in the model (Fig. 3). While the overall NRE indicates significant agreement, the NRE itself has a tendency to have significant changes from extreme positive to extreme negative values (i.e., the low overall NRE is due to error cancellation). Consistent with the equilibrium-based calculations discussed above, the calculated fugacities35 in the sampler and ambient air over the course of the simulation (data not shown) indicate that the XAD-PAS should depurate these chemicals only towards the end of the simulation (days 283–365), when the concentration of these chemicals in ambient air concentration becomes negligible. To explore the hypothesis of degradation within the sampler,32 a 1st-order degradation rate constant applied to the sorbed phase (i.e., kdeg-PAS, d−1) was introduced and fitted until the simulation shape resembled the experimental data obtained. In both cases, a rate constant of 0.0125 d−1 produced acceptable results with respect to the temporal trends in the empirical uptake curves.
PSR estimation and model performance
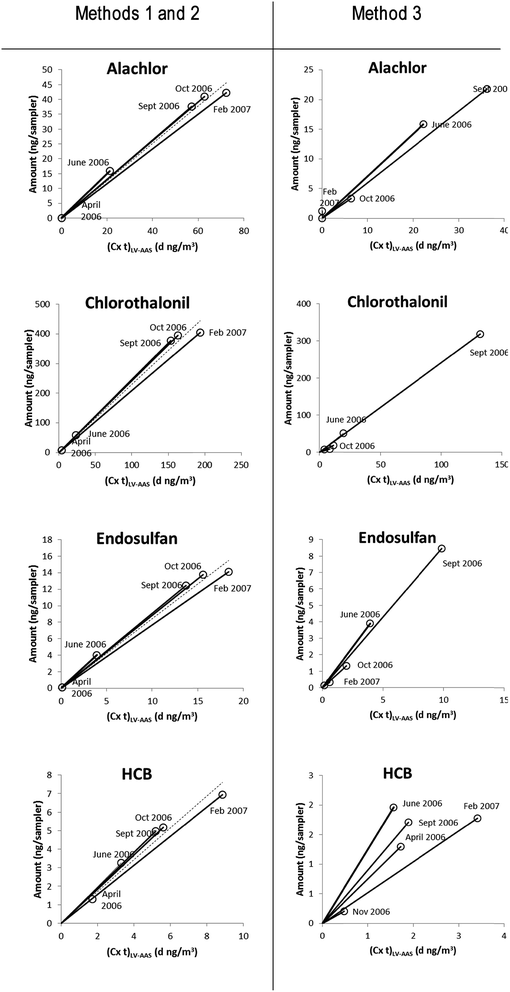
Several methods were used to estimate the PSR of the XAD-PAS. A summary of the results can be found in Table 1. Some examples depicting the values of PSRE given by the slopes are shown in Fig. 4. Detailed results of each calculation method described in the Methods section above can be found in the ESI, Section S7 to S9.† Note that PSRs were not calculated for pendimethalin and trifluralin because of the discrepancy between the LV-AAS and empirical XAD-PAS data.| Compound | PSRE (m3 d−1) | PSRW (m3 d−1) | ||
|---|---|---|---|---|
| Method 1 | Method 2 | Method 3 | ||
| Alachlor | 0.66 ± 0.07 | 0.63 ± 0.02 | 0.61 ± 0.09 | 0.54 |
| Atrazine | 0.72 ± 0.06 | 0.70 ± 0.03 | 0.66 ± 0.10 | 0.61 |
| Chlorothalonil | 2.27 ± 0.30 | 2.29 ± 0.09 | 1.90 ± 0.62 | 0.67 |
| cis-Chlordane | 0.48 ± 0.06 | 0.43 ± 0.02 | 0.42 ± 0.10 | 0.50 |
| trans-Chlordane | 0.56 ± 0.03 | 0.55 ± 0.01 | 0.54 ± 0.07 | 0.51 |
| DCPA | 0.47 ± 0.09 | 0.42 ± 0.03 | 0.35 ± 0.13 | 0.57 |
| Disulfoton | 0.68 ± 0.05 | 0.67 ± 0.03 | 0.65 ± 0.19 | 0.53 |
| Endosulfan I | 0.89 ± 0.09 | 0.84 ± 0.04 | 0.78 ± 0.19 | 0.54 |
| Endosulfan II | 0.67 ± 0.06 | 0.65 ± 0.03 | 0.62 ± 0.15 | 0.53 |
| Endosulfan sulfate | 0.31 ± 0.01 | 0.31 ± 0.01 | 0.34 ± 0.06 | 0.55 |
| HCB | 0.88 ± 0.10 | 0.86 ± 0.04 | 0.77 ± 0.33 | 0.67 |
| α-HCH | 1.15 ± 0.18 | 1.06 ± 0.07 | 0.99 ± 0.32 | 0.64 |
| γ-HCH | 0.93 ± 0.16 | 0.91 ± 0.04 | 0.88 ± 0.31 | 0.63 |
| Metolachlor | 0.74 ± 0.04 | 0.72 ± 0.02 | 0.68 ± 0.10 | 0.50 |
| trans-Nonachlor | 0.43 ± 0.04 | 0.41 ± 0.02 | 0.39 ± 0.08 | 0.52 |
The empirical PSR values (PSRE) for HCB, α-HCH and γ-HCH agree very well with values that were determined previously in the same region (Burnt Island and Point Petre, in central and southern Ontario, respectively).16 In contrast, the values are higher than those obtained under Arctic conditions (Alert),16 and lower than those from Costa Rica,7 confirming the need for temperature-dependent calibration. These results support the use of Method 3 as an accurate way to obtain PSRE, despite its associated variability which is higher than the uncertainty obtained for the other two methods, largely because it accounts for the temperature-dependent variation in PSRE. The ratio of the average PSRE during the Spring–Summer (end of April–August) and Fall–Winter (March–April, September–February) periods, PSRsummer and PSRwinter, respectively (data shown in the ESI, Section S9†), indicates that the sampling rates are an average of 40% higher during the warmest deployment periods. Thus Method 3 is likely the best estimate of the XAD-PAS sampling rates over the entire year, as it most accurately accounts for the seasonal variations throughout the deployment. However, as can be seen in Table 1, differences in the estimated PSREs via Methods 1–3 are often less than the associated uncertainties in the estimates and therefore, in practical terms, data availability (i.e., sampling intervals over deployment period) is the key factor.
The wind speed adjusted sampling rates estimated by the PAS-SIM model for a 10 mm stagnant boundary layer (PSRW) also are presented in Table 1. As shown, the PSRW agrees with the empirical sampling rates derived from the field deployment in Egbert for the majority of chemicals, following from the model performance illustrated in Fig. 1–3. Note that the modeled PSRW is an inherent sampling rate that is independent of the concentration of the chemical in the air and amount of chemical on the sampler and only the sampler dimensions, meteorological conditions and the physicochemical properties are taken into account to calculate it.17 Because the information needed to calculate PSRW over time is often available, it can be estimated for any site prior to and during the deployment period to inform the interpretation of empirical XAD-PAS data. Such calculations could be particularly useful to probe site-to-site and year-to-year variations in passive air monitoring data. Parameterization of the model with the most detailed meteorological records available (e.g., temperature and wind speed at daily resolution) is recommended for this purpose, especially for sites experiencing substantial weather variability.
Conclusions
The main objective of this study was to evaluate the performance of the PAS-SIM model using a passive air sampler calibration study for pesticides. Considering the potential uncertainty in input parameters (i.e., the LV-AAS data and partitioning property values) and empirical XAD-PAS data, the PAS-SIM model performed reasonably well for the majority of chemicals simulated in the study (e.g., FoA within a factor of two). While other model evaluations for any additional chemicals would be valuable, the relatively poor model performance for chlorothalonil, trifluralin and pendimethalin in particular demands further analyses. The apparent discrepancy between the LV-AAS and empirical XAD-PAS data for trifluralin and pendimethalin is of most interest because of the possibility that degradation of compound sorbed to the XAD resin is a key consideration, as was reported for chlorpyrifos.32 For the other compounds simulated, the model evaluation suggests that the PAS-SIM model can be used to characterize the expected XAD-PAS sampling rates (PSRW) at any site for which meteorological data are available. Overall, the findings allow us to conclude that PAS-SIM is a useful modeling tool for pesticides that can enable a better understanding of PAS uptake kinetics under varying ambient air concentrations and meteorological conditions and provide insights facilitating an improved interpretation of empirical XAD-PAS data. Application of the model prior to actual deployment of XAD-PAS may also allow researchers to develop sampling strategies more appropriate for the target analytes of interest and purposes of the monitoring campaign.A secondary objective was to further assess the appropriateness and applicability of three methods for deriving empirical sampling rates from calibration studies. The selection of the most appropriate approach to quantify PSR depends on the availability of the data and the accuracy needed. Simple approaches (i.e., Method 1 or 2) may be sufficient to estimate the empirical sampling rate (PSRE) if the weather conditions or ambient concentrations are expected to be relatively stable over the deployment period. When a site is expected to have strong seasonal or meteorological variability, Method 3 will likely yield a better estimate and is recommended if PAS were deployed and collected at appropriate intervals. While empirical PSREs for target compounds from a given site could be assumed to be valid for other sites with similar meteorological conditions, a more rigorous approach would be to use all available literature data to make an estimate by linear regression of the experimental PSR against a site-specific characteristic, e.g., temperature.36 Using multiple literature values has a real advantage over the extrapolation of a single empirical sampling rate. The main limitation for this approach may be the lack of data needed and/or the lack of congruence across that data. As noted in the Methods section, the use of depuration compounds has been promoted as a method to estimate PSRs in the absence of concurrent AAS data but is subject to some uncertainty.21,22 Although outside the scope of the current study, the PAS-SIM model can also be used to simulate the behaviour of depuration compounds under different meteorological conditions. Simulated PSRs based on uptake scenarios and derived from depuration scenarios could then be compared and used to gain insight into potential error associated with the use of depuration compounds to estimate PSR using the current approach. Such simulations are considered a priority for future applications of the PAS-SIM model. Development of a PAS-SIM parameterization set for simulating the uptake of organic compounds on PUF-PAS is also desirable, given the widespread use of this type of PAS for field deployments.
Acknowledgements
We are grateful to the Government of Canada's Emerging Leaders in the Americas Program (ELAP) for a scholarship to ARR and the Natural Sciences and Engineering Research Council of Canada (NSERC) and the Canadian Foundation for Climate and Atmospheric Sciences (CFCAS) for funding. The PAS-SIM model is freely available by request to the Corresponding Author.References
- D. C. G. Muir, C. Teixeira and F. Wania, Environ. Toxicol. Chem., 2004, 23, 2421–2432 CrossRef CAS.
- L. Shen, F. Wania, Y. D. Lei, C. Teixeira, D. C. G. Muir and T. F. Bidleman, Environ. Sci. Technol., 2005, 39, 409–420 CrossRef CAS.
- G. L. Daly, Y. D. Lei, C. Teixeira, D. C. G. Muir and F. Wania, Environ. Sci. Technol., 2007, 41, 6020–6025 CrossRef CAS.
- Y. Yao, T. Harner, P. Blanchard, L. Tuduri, D. Waite, L. Poissant, C. Murphy, W. Belzer, F. Aulagnier and E. Sverko, Environ. Sci. Technol., 2008, 42, 5931–5937 CrossRef CAS.
- S. J. Hayward, T. Gouin and F. Wania, J. Agric. Food Chem., 2010, 58, 1077–1084 CrossRef CAS PubMed.
- T. Gouin, L. Jantunen, T. Harner, P. Blanchard and T. Bidleman, Environ. Sci. Technol., 2007, 41, 3877–3883 CrossRef CAS.
- T. Gouin, F. Wania, C. Ruepert and L. E. Castillo, Environ. Sci. Technol., 2008, 42, 6625–6630 CrossRef CAS.
- M. Shoeib and T. Harner, Environ. Sci. Technol., 2002, 36, 4142–4151 CrossRef CAS.
- C. Moeckel, T. Harner, L. Nizzetto, B. Strandberg, A. Lindroth and K. C. Jones, Environ. Sci. Technol., 2009, 43, 3227–3232 CrossRef CAS.
- P. Bohlin, K. C. Jones and B. Strandberg, Environ. Sci. Technol., 2010, 44, 749–754 CrossRef CAS PubMed.
- S. J. Hayward, T. Gouin and F. Wania, Environ. Sci. Technol., 2010, 44, 3410–3416 CrossRef CAS PubMed.
- S. Hazrati and S. Harrad, Chemosphere, 2007, 67, 448–455 CrossRef CAS PubMed.
- L. Tuduri, T. Harner and H. Hung, Environ. Pollut., 2006, 144, 377–383 CrossRef CAS PubMed.
- J. Klanova, P. Eupr, J. Kohoutek and T. Harner, Environ. Sci. Technol., 2008, 42, 550–555 CrossRef CAS.
- X. Zhang, T. N. Brown, A. Ansari, B. Yeun, K. Kitaoka, A. Kondo, Y. D. Lei and F. Wania, Environ. Sci. Technol., 2013, 47, 7868–7875 CrossRef CAS PubMed.
- F. Wania, L. Shen, Y. D. Lei, C. Teixeira and D. C. G. Muir, Environ. Sci. Technol., 2003, 37, 1352–1359 CrossRef CAS.
- J. M. Armitage, S. J. Hayward and F. Wania, Environ. Sci. Technol., 2013, 47, 13546–13554 CrossRef CAS PubMed.
- L. Melymuk, M. Robson, P. A. Helm and M. L. Diamond, Atmos. Environ., 2011, 45, 1867–1875 CrossRef CAS PubMed.
- C. Persoon and K. C. Hornbuckle, Chemosphere, 2009, 74, 917–923 CrossRef CAS PubMed.
- L. Tuduri, M. Millet, O. Briand and M. Montury, TrAC, Trends Anal. Chem., 2012, 31, 38–49 CrossRef CAS PubMed.
- X. Zhang, M. Tsurukawa, T. Nakano, Y. D. Lei and F. Wania, Environ. Sci. Technol., 2011, 45, 10509–10515 CrossRef CAS PubMed.
- X. Zhang and F. Wania, Environ. Sci. Technol., 2012, 46, 9563–9570 CrossRef CAS PubMed.
- EU Reference Laboratories for Residues of Pesticides, EURL DataPool, http://www.eurl-pesticides-datapool.eu/.
- M. H. Abraham, K. Enomoto, E. D. Clarke and G. Sexton, J. Org. Chem., 2002, 67, 4782–4786 CrossRef CAS PubMed.
- M. H. Abraham, K. Enomoto, E. D. Clarke, M. Roses, C. Rafols and E. Fuguet, J. Environ. Monit., 2007, 9, 234–239 RSC.
- H. P. H. Arp, R. P. Schwarzenbach and K.-U. Goss, Environ. Sci. Technol., 2008, 42, 5951–5957 CrossRef CAS.
- H. C. Tülp, K.-U. Goss, R. P. Schwarzenbach and K. Fenner, Environ. Sci. Technol., 2008, 42, 2034–2040 CrossRef.
- L. M. Sprunger, S. S. Achi, W. E. Acree Jr and M. H. Abraham, Fluid Phase Equilib., 2010, 288, 139–144 CrossRef CAS PubMed.
- S. J. Hayward, Y. D. Lei and F. Wania, Atmos. Environ., 2011, 45, 296–302 CrossRef CAS PubMed.
- M. MacLeod, M. Scheringer and K. Hungerbühler, Environ. Sci. Technol., 2007, 41, 2827–2832 CrossRef CAS.
- S. Ellison and A. Williams, Eurachem/CITAC guide: Quantifying Uncertainty in Analytical Measurement, 3rd edn, 2012 Search PubMed.
- J. L. Armstrong, R. A. Fenske, M. G. Yost, M. Tchong-French and J. Yu, Chemosphere, 2013, 92, 451–457 CrossRef CAS PubMed.
- P. Bohlin, O. Audy, L. Skrdlikova, P. Kukucka, P. Pribylova, R. Prokes, S. Vojta and J. Klanova, Environ. Sci.: Processes Impacts, 2014, 16, 433–444 CAS.
- L. Melymuk, P. Bohlin, O. Sanka, K. Pozo and J. Klanova, Environ. Sci. Technol., 2014, 48, 14077–14091 CrossRef CAS PubMed.
- D. Mackay, Multimedia Environmental Models - The Fugacity Approach, CRC Press LLC, Boca Raton, FL, USA, 2nd edn, 2001 Search PubMed.
- X. P. Wang, P. Gong, T. D. Yao and K. C. Jones, Environ. Sci. Technol., 2010, 44, 2988–2993 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5em00122f |
| This journal is © The Royal Society of Chemistry 2015 |