Gd(III) chelates for MRI contrast agents: from high relaxivity to “smart”, from blood pool to blood–brain barrier permeable
Chang-Tong
Yang
* and
Kai-Hsiang
Chuang
Laboratory of Molecular Imaging, Singapore Bioimaging Consortium, Agency for Science, Technology and Research (A*STAR), 11 Biopolis Way, #02-02 Helios, Singapore 138667. E-mail: yang_changtong@sbic.a-star.edu.sg; Fax: +65-64789957; Tel: +65-64788727
First published on 7th February 2012
Abstract
Paramagnetic Gd(III) chelate contrast agents have been extensively used to enhance the signal of MRI scans for the last three decades. The use of Gd(III) chelate contrast agents is projected to increase as new agents and applications arise, thanks to the favorable combination of a large magnetic moment and long electron spin relaxation time of the Gd(III) ion. The relaxivity and stability of Gd(III) chelates are the primary requisites for the development of contrast agents as both small doses and low release of free Gd(III) ions will reduce the toxicity. The physico-chemical parameters and structure-related relaxation mechanisms provide the strategies for chelate design. The higher relaxivity and efficacy of the contrast agent can be improved by designing pH-, metal ion-, enzyme- or small biomolecule-dependent “smart” contrast agents. Through conjugation to biomacromolecules such as polymers, dendrimers or non-covalent binding to plasma proteins, contrast agents could increase the blood half-life and can be used for contrast-enhanced MRI. When conjugated to certain diagnostic or therapeutic proteins, low molecular weight Gd(III) chelates could cross the blood–brain barrier (BBB) by means of a receptor-mediated transport system or receptor-mediated transcytosis.
 Chang-Tong Yang | Chang-Tong Yang received PhD in chemistry at National University of Singapore in 2003. He took up several postdoc positions at The Universities of Iowa, University of Michigan and Purdue University where he conducted research on preparation of polylanthanide complexes with amino-acids as MRI contrast agents and development of 64Cu-labeled target-specific radiopharmaceuticals with new bifunctional chelators for PET imaging. He joined the Singapore Bioimaging Consortium, A*STAR in 2007, working on several projects on probe developments for MRI, SPECT and PET. He became project leader of Intramural and Joint Research Council projects from A*STAR. |
 Kai-Hsiang Chuang | Kai-Hsiang Chuang received Ph.D. in electrical engineering from National Taiwan University, Taipei, Taiwan in 2001, where he studied methods for detecting brain function using magnetic resonance imaging (MRI). He did postdoc in the National Institutes of Health, USA till 2007 where he focused on contrast enhanced methods especially using manganese as a functional contrast agent in the brain. He joined the Singapore Bioimaging Consortium as the head of MRI Group since 2008. His research interest is on the development of functional imaging methods, including pulse sequences and contrast agents, for early detection of neurodegenerative diseases and cancers. |
1. Introduction
Magnetic resonance imaging (MRI) has revolutionized diagnostic medicine by providing a non-invasive imaging modality that complements computed tomography (CT) and nuclear medicine techniques without ionizing radiation.1 MRI contrast agents are diagnostic magneto-pharmaceuticals usually containing low molecular weight Gd(III) chelates with an acyclic or macrocyclic ligand.2–5 The use of contrast agents is projected to increase as new contrast agents and applications arise. Gadolinium(III) is the constituent of most MRI contrast agents, thanks to its favorable combination6 of a large magnetic moment (spin-only μeff = 7.94 BM, from seven half-filled f orbitals) and long electron spin relaxation time (10−8 to 10−9 s, from symmetric S electronic state). All acyclic and macrocyclic Gd(III) MRI contrast agents relax inner sphere and/or outer sphere waters. Macrocyclic-based systems have a number of pharmacological advantages because of the tight binding of the macrocycle, but macrocycles decrease or eliminate inner sphere water sites. Good contrast agents must have ideal physicochemical, pharmacological, and radiological properties7 including being nonionic to minimize osmolality and having good water solubility, high relaxivity, thermodynamic and kinetic stability, in vivo stability, low toxicity, etc.This review will first focus on the relaxivity of Gd(III) chelate contrast agents and several parameters that influence the relaxivity. The relaxivity and stability of Gd(III) chelates are the primary requisites for the development of contrast agents. The physico-chemical parameters and structure-related relaxation mechanisms provide the strategies for chelate design. The higher relaxivity and efficacy of contrast agents can be improved by designing pH-, metal ion-, enzyme- or small biomolecule-dependent “smart” contrast agents. The mechanisms underlying the development of “smart” contrast agents thus will be addressed. Through conjugation to biomacromolecules such as polymers, dendrimers or non-covalent binding to plasma proteins, contrast agents could increase the blood half-life and can be used for contrast-enhanced MRI. And through conjugation to certain diagnostic or therapeutic proteins, low molecular weight Gd(III) chelates could cross the blood–brain barrier (BBB) by means of a receptor-mediated transport system or receptor-mediated transcytosis. We summarize blood pool contrast agents and BBB permeable contrast agents by discussing the structures of published compounds. Rather than concentrating on one aspect of contrast agents such as low molecular weight Gd(III) chelates2,4 or ligand design,8 the purpose of this review is to demonstrate the major developments for Gd(III) chelates as MRI contrast agents from high relaxivity to “smart”, from blood pool to blood–brain barrier. It provides some future research directions for this emerging field to those who are experts in coordination chemistry, polymer and supramolecular chemistry, medicinal chemistry, spectroscopy, biology, and radiology.
2. Relaxivity of Gd(III) based MRI contrast agents
With the assistance of a contrast agent and a better insight of structure-related relaxation mechanisms, image contrast could be improved greatly. Inorganic chemists have played a central role in the development of low molecular weight Gd(III) contrast agents, and the design of monomeric Gd(III) contrast agents is a mature field.2,4,8 The proton relaxivity of a Gd(III) compound is determined by several factors and can be varied depending on magnetic field strength, viscosity, and temperature. The T1 relaxivity (r1) for a low molecular weight Gd(III) chelate is 3–5 mM−1 s−1. The equation9–11r1 = Cqμeff2τcr−6 expressed the principal factors that influence the relaxivity under ambient conditions, in which C is a constant, q is the number of inner sphere water molecules, μeff is the effective magnetic moment, τc is the molecular correlation time, and r is the Gd⋯H (H2O) distance. Among them, inner-sphere water protons are most effectively relaxed because of the r−6 dependence.The molecular correlation time τc is determined by the following parameters: rotational correlation time τr, the electronic correlation time τs, and the proton residence time τm (i.e., reciprocal of the water exchange rate) as expressed in the equation τc−1 = τr−1 + τs−1 + τm−1. Theory shows that maximum relaxivity occurs when the dipole–dipole correlation time is the inverse of the proton Larmor processional frequency, with optimal τc1s of 7.4 ns at 0.5 T and 2.5 ns at 1.5 T. The zero-field value of the electronic relaxation time (τs0) is important at clinically relevant fields,11 with τr and τs0 accounting for relaxivity differences. The τs0 is related to Δ2 and τv by the relation τs0 = (12Δ2τv)−1 (τv, the correlation time of the fluctuation of the transient zero-field splitting; Δ, the trace value).12 The τs0 value reflects the coordination symmetry and the nature of substituents, for example, amidation of a ligand carboxy group decreases τs0 dramatically and results in a lower water proton relaxivity at low fields.
There is considerable room for improvement of τr and τm values. The rotational correlation time of simple Gd(III) chelates is too fast (∼10−10 s), slowing it to 10−8 s would improve relaxivity. Changing molecular size is one of the possible approaches to increase τr. This is the basis for macromolecular and targeted contrast agents in which Gd(III) chelates are covalently attached to each other or to conjugate molecules such as polymers, dendrimers, or biomacromolecules.13–16 Increases in τr can lead to dramatic increases in r1; for example, protein-bound Gd(DTPA) has relaxivities approaching 20 mM−1 s−1, compared to 4 mM−1 s−1 for the chelate alone. While increases in τr can bring substantial increases in relaxivity, ligand design is needed to ensure that water exchange is not so slow as to offset gains from τr increases. The proton residence time is assumed to be equal to the water residence time since proton exchange at physiological pH is determined by water exchange. For the Gd(III) chelates with one water molecule coordinated to Gd(III) (q = 1), the exchange rate is dependent on the residual electric charge and the structural properties of the complex. The exchange rate is a function of the difference in energy between the 9-coordinate ground state and the 8-coordinate intermediate in dissociative water exchange, so bulky macrocycle substituents can cause a decrease in ground-state stability.17–20 The main issues with macromolecular and dendrimeric contrast agents are the slow rate of water exchange and the lack of correlation between rotations of the macromolecule and of the Gd⋯H vector.21
High relaxivity is the primary requisite for the development of molecular imaging MR probes and targeting probes.22–25 Several strategies for increasing the sensitivity (relaxivity) of gadolinium-based MRI contrast agents have been reviewed by Caravan.26 Some Gd(III) contrast agent candidates with enhanced relaxivity have been reported, including (1) a HOPO Gd(III) complex with an r1 of 10.5 mM−1 s−1;27 (2) a texaphyrin Gd(III) complex with a high r1 of 16.9–19 mM−1 s−1 (although it drops to 5.3 mM−1 s−1 in phosphate buffer);28 (3) a β-cyclodextrin “click cluster” decorated with seven paramagnetic Gd(III) chelates with a high r1 of 43.4 mM−1 s−1 per molecule at 9.4 T;29 (4) a tetranuclear Gd(III) complex of DO3A appended onto the pentaerythrityl framework with a high r1 of 28.13 mM−1 s−1 (24 MHz, 35 °C, pH 5.6).30 The theoretical estimate of the maximum proton relaxivities of 80–100 mM−1 s−1 based on the Solomon–Bloembergen–Morgan equations for a monomeric Gd(III) chelate is much higher than the average 4 mM−1 s−1 of current agents that require concentrations of 0.1 mM,31–34 when the three most important influencing factors—the rotation, the electron spin relaxation and the water exchange rate—are simultaneously optimized. Thus, there is very considerable room for improvement in relaxivity.
3. Smart contrast agents
Smart MRI contrast agents are agents that undergo a large change in relaxivity upon various stimuli. Specific physiological or biochemical processes such as changes in pH,35–47 metal ions,57–79 enzymatic activity,80–87 and small biomolecules90–93 could change the coordination of the Gd(III) sphere in the Gd(III) chelates, and change the relaxivity of contrast agents accordingly because of the effect of the number of coordinated water molecules, the water exchange rate and the rotational correlation time. The smart contrast agents have been reviewed by Desreux,94 Lowe,95 Strijkers,96 and Geraldes.97 We summarize here the Gd(III) chelates as smart contrast agents with some recently reported examples.A. pH-activated contrast agents
Sherry et al. reported the first example of a novel pH-sensitive MRI contrast agent.35 The r1 relaxivity of the Gd3+ complex of a DOTA tetraamide derivative, GdDOTA-4AmP5− (Fig. 1), has an unusual pH dependence. The relaxivity r1 increases from pH 4 to a maximum near pH 6, and gradually decreases to a minimum near pH 8.5, then increases again until at pH 10.5. Further studies showed that GdDOTA-4AmP5− only has such pH-sensitive property when the complexation reaction is conducted at a pH above 8. Because of the amphoteric character of the phosphonate side chains, rapid prototropic exchange between the single coordinated water and bulk water contributes to the pH-dependent relaxivity.36 The unique pH-dependence of relaxivity has its application in in vivo tissue pH imaging and extracellular pH imaging.37–39 When the phosphonates were replaced by a hydroxypyridyl group, the compound showed two regions of enhanced relaxivity.40 The small relaxivity enhancement (25%) at pH 2–4 is because of an increase in the prototropic exchange of coordinated water. The relaxivity enhancement at pH 6–9 is attributed to the deprotonation of the ligand amide protons that are involved in an intramolecular acid–base pair interaction with the phenolic protons and thus results in the formation of a second hydration sphere. Based on the established pH-sensitive characteristic of GdDOTA-4AmP5−, Caravan et al. modified the structure by employing click chemistry to incorporate a fluorine atom (either 18F or 19F) to make this complex GdDOTA-4AmP-F (Fig. 2) a smart bimodal MR-PET (PET = positron emission tomography) agent for quantitative pH imaging.41 This strategy offers an approach to develop other smart bimodal MR-PET probes.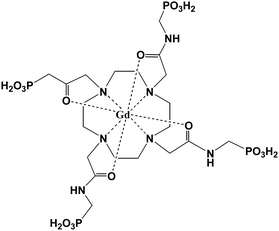 | ||
| Fig. 1 Structure of GdDOTA-4AmP5− (adapted from ref. 35). | ||
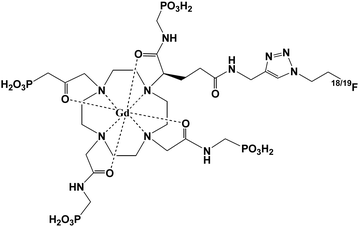 | ||
| Fig. 2 Structure of GdDOTA-4AmP-F (adapted from ref. 41). | ||
Aime and coworkers reported the macromolecule containing thirty Gd(III) chelates conjugated to a poly(amino acid) chain consisting of 114 ornithine residues that undergoes relaxation change by protonation and deprotonation.42 At pH < 4, the protonated amino groups of polypeptide in (GdDO3ASQ)30Orn114 are highly hydrated and the Gd chelates maintain a high degree of mobility. With an increase of pH, the progressive deprotonation of the NH3+ groups leads to the formation of intramolecular hydrogen bonds between adjacent peptide linkages. The structural changes limit the mobility of the chelate moieties and result in higher relaxivity due to an increase of τR of the molecule.
Reversible intramolecular and intermolecular anion binding by Gd(III) complexes of seven-coordinate DOTA-triamide ligands is a novel approach to design pH-activated agents. In acidic solutions, these Gd(III) complexes contain two inner-sphere water molecules (q = 2) to reach a nine-coordination number in Gd(III) and hence had a much higher relaxivity. While in basic solutions with a pH increase, anion media like hydrogencarbonate43 or intramolecular deprotonated sulfonamide group nitrogen44 will progressively replace the two aqua ligands, leading to a decrease of relaxivity.
Relaxation changes of Gd(III) complexes can be caused not only by protonation or deprotonation of the complex, by changes in the configuration of the complex, but also by the formation of aggregates as a consequence of pH as well.45–47 It was found that polyethylene glycol (PEG)-grafted DPPE (dipalmitoyl-phosphatidylethanolamine)/DPSG (dipalmitoyl-glycerosuccinate) liposomal GdDTPA-BMA (gadodiamide, gadolinium diethylenetriamine pentaacetic acid bismethylamide) was stable in blood at physiological pH and displayed a remarkable pH sensitivity.46 An in vitro MRI phantom study showed the potential value of the DPPE/DPSG liposomal GdDTPA-BMA system as a probe for monitoring pH.
These reported pH-activated contrast agents have been focused to achieve the higher relaxivity by various approaches. The limitation is that only a few of these contrast agents are applicable to an in vivo study. For example, the pH-sensitive contrast agent GdDOTA-4AmP5− has been used to map pH values in vivo.48,49 The slow development of the smart contrast agents can be explained by the fact that the concentration of the agent will change with time and vary in different tissues in vivo. Unlike in vitro, the concentration of the agent is known and keeps constant. One of the major challenges in this field is designing the more sensitive pH-responsive contrast agents within the physiological pH condition. Another challenge is to develop tissue pH biomarkers for identifying disease and evaluation of response to therapy.
Recently, water-soluble gadonanotube derivatives that exhibited a dramatic response to pH change under physiologically relevant conditions were reported by Wilson et al.50 Though it is outside the scope of this review because gadonanotubes are Gd-containing metallofullerenes but not Gd(III)-chelates, it is worth mentioning here. The relaxivity of the gadonanotubes undergoes a dramatic increase from 40 mM−1 s−1 (pH = 8.3) to 133 mM−1 s−1 (pH = 6.7) at 37 °C with approximately 40 mM−1 s−1 change between pH 7.4 and 7.0. The result suggested that gadonanotubes might be excellent candidates for the development of clinical agents for the early detection of cancer since the extracellular pH of cancerous tissues is less than 7.0 and in some cases as low as 6.3 (ref. 39) while the normal physiological pH is 7.4. Some gadofullerene derivatives including Gd@C60(OH)X, Gd@C60[C(COOH)2]10 and Gd@C82(OH)X are reported to have good relaxivity as potential MRI contrast agents.51–56 With the dramatic response to pH around physiological pH, the gadonanotubes have potential applications in MRI contrast agent development.
B. Metal ion-activated contrast agents
Meade and coworkers developed a calcium(II) ion dependent MRI contrast agent.57,58 By incorporating a Ca(II) binding into a Gd(III) complex with a DOPTA ligand system (Fig. 3), the relaxivity of the complex increased 75% from 3.3 mM−1 s−1 to 5.8 mM−1 s−1 (500 MHz, 298 K). The significant increase was attributed to a substantial conformational change of the Gd complex when Ca2+ is bound, and changed the longitudinal relaxation time of water protons. Since then, a series of metal ion-responsive smart contrast agents have been designed and synthesized. Chang and coworker59 have reviewed the metal ion responsive smart contrast agents in detail, including Ca2+,57,58,60–63 K+,64 Mg2+,64 Zn2+,65–71 Fe2+,72–76 Cu2+,77 Cu+-sensitive agents,78,79 and summarized the general design criteria for preparing such metal-ion responsive MRI contrast agents and their applications in biological systems. | ||
| Fig. 3 Scheme of complex DOPTA-Gd (adapted from ref. 57). | ||
Sherry et al.65 reported a first-generation paramagnetic CEST agent, [Eu(dotampy)] (dotampy = 1,7-bis(N,N-bis(2-pyridylmethyl)-aminoethylcarbamoylmethyl)-4,10-bis(butylcarbamoylmethyl)-1,4,7,10-tetraazacyclododecane), as a novel sensor for Zn2+ ions. Adding Zn2+ to [Eu(dotampy)] in buffered solution at pH 7.1 will cause broadening of the resonances of both the Eu3+ bound water molecule and bulk water in the CEST spectrum, indicating a more rapid water exchange. And water exchange is even faster when Zn2+ ions were added to [Eu(dotampy)] at pH 8.0 so that the resonance of Eu3+ bound water disappeared. The authors proposed the possibility that Zn2+ may have a coordinated water molecule that is partially deprotonated at these pH values, leading to a Zn2+OH species near the Eu3+-bound water molecule (Fig. 4). Such a species could favor the exchange rate between the Eu3+ bound water molecule and the bulk water.
![Hypothetical structures of [Eu(dotampy)] in the presence and absence of Zn2+ ions (adapted from ref. 65).](/image/article/2012/MD/c2md00279e/c2md00279e-f4.gif) | ||
| Fig. 4 Hypothetical structures of [Eu(dotampy)] in the presence and absence of Zn2+ ions (adapted from ref. 65). | ||
Iron–gadolinium heterobimetallic complexes as high relaxivity contrast agents were developed by Desreux and others.72–76 Complex Gd-PhenHDO3A (PhenHDO3A = rel-10-[(5R,6R)-5,6-dihydro-6-hydroxy-1,10-phenanthrolin-5-yl]-1,4,7,10-tetraazacyclododecane-1,4,7-triacetic acid)72,73 (Fig. 5) forms a supramolecule with iron by self-assembly to get an increase of relaxivity from 5.1 to 12.5 mM−1 s−1 (20 MHz, 298 K) due to an increase of the molecular weight and longer rotational correlation times (τR). The same strategy was employed for other high relaxivity Fe–Gd heterobimetallic complexes, with one Fe2+ center linking multiple Gd3+-chelates to form supramolecular assemblies with increased molecular weight.74–76
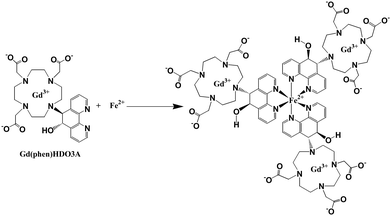 | ||
| Fig. 5 Self-assembly of Gd-PhenHDO3A around Fe(II), resulting in an increased relaxivity (adapted from ref. 72). | ||
Recently Chang and coworker77 designed a smart MRI contrast agent for selective Cu2+ sensing. The smart MRI contrast agent is complex Gd-DO3A with a pendant iminodiacetate site for binding Cu2+. In the absence of Cu2+, the pendant receptor will block inner-sphere water from accessing Gd3+ and thus decrease relaxivity (3.76 mM−1 s−1) (400 MHz, 298 K) whereas the addition of Cu2+ triggers a 41% relaxivity enhancement to 5.29 mM−1 s−1 because binding of Cu2+ to the pendent receptor clears the way for water to access the inner-sphere and subsequently increases the proton relaxivity (Fig. 6). A similar approach was used to design the Cu(I)-activated Gd-chelate contrast agent by the same group.76,77
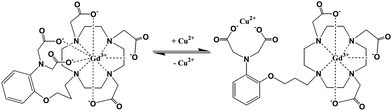 | ||
| Fig. 6 Proposed action for sensing Cu2+ (adapted from ref. 77). | ||
C. Enzyme-activated contrast agents
One of the strategies for designing an enzyme-activated contrast agent is using a contrast agent to detect regions in the body or cells on which some specific enzymes are focused. The MRI contrast agent (4,7,10-tri(acetic acid)-1-(2-β-galactopyranosylethoxy)-1,4,7,10-tetraaza-cyclododecane)gadolinium (EGad) has a galactopyranose residue at the ninth coordination site of the Gd3+ ion.80,81 Once the EGad is exposed to the commonly used marker enzyme β-galactosidase (β-gal), the β-galactose moiety will be cleaved and replaced by water which will increase the relaxivity (Fig. 7). When R = H, the relaxivity of the Gd(III) complex increased by ∼20%, while when R = Me (methyl group), it increased significantly by 200%. The system demonstrated the concept of using MR imaging for in vivo visualization of gene expression. It is also an example of monitoring of enzyme activity by modulation of hydration state. A new β-galactopyranose-containing Gd3+ complex (Gd(DOTA-FPG)(H2O)) was designed and observed to be bioactivated.82 The Gd(DOTA) chelate was linked to a bioactivated residue β-D-galactopyranose, which can be activated by β-galactosidase. The enzymatic moiety, 2-difluoromethylphenyl-β-galactopyranoside, can be cleaved when there is an existence of β-galactosidase. The r1 relaxivity increased significantly the enzymatic cleavage of [Gd(DOTA-FPG)(H2O)] in the presence of β-galactosidase and HSA, meaning that the HSA and β-galactosidase conjugate to the Gd(III) chelate after the galactopyranoside residue is removed. The in vivo studies demonstrated that the signal intensities of tumors with β-galactosidase gene expression are significantly higher than those of tumors without β-galactosidase gene expression. Bertini and coworkers reported that Gd-DTPA was functionalized with a sulfonamide (SA) (Fig. 8), which was designed to selectively target the enzyme carbonic anhydrase, binds carbonic anhydrase effectively and certainly showed a significant relaxation enhancement.83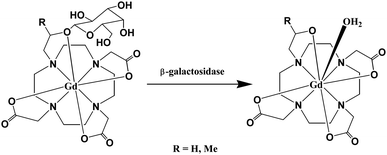 | ||
| Fig. 7 Schematic of the transition of EGad from a weak to a strong relaxivity state (adapted from ref. 80). | ||
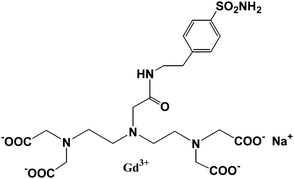 | ||
| Fig. 8 Scheme of Gd-DTPA-SA (adapted from ref. 83). | ||
An esterase-activated contrast agent was reported by Lowe et al.84 A cyclen-based Gd(III) complex with pendant acetoxymethyl esters (Fig. 9) is seven-coordinated with ligands and has two inner-sphere water molecules to saturate the nine-coordinated numbers. However, the complex did not show high relaxivity due to its affinity to endogenous serum anions such as HCO3−. The anions may replace these two water molecules leading to a poor contrast agent. By using enzyme-activation, the complex will switch from a neutral contrast agent with carbonate-binding to a negatively charged one and result in a change in hydration state. On activation by porcine liver esterase, the relaxivity of the Gd(III) complex increased by ∼85% at physiological pH and NaHCO3, as anion binding suppressed negatively charged species.
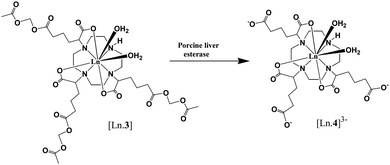 | ||
| Fig. 9 Schematic of activation by porcine liver esterase (adapted from ref. 84). | ||
The other approach is the binding of the agent to a macromolecule, such as HSA binding, or polymerization of the contrast agent itself, which substantially slows molecular rotation of the Gd3+ complex, resulting in an additional increase in the relaxivity and tissue contrast known as the receptor-induced magnetization enhancement (RIME) approach. A tri-lysine peptide was chosen to conjugate to a GdDTPA derivative as it has a poor affinity for HSA binding and can be cleaved by a human carboxypeptidase B, Thrombin Activatable Fibrinolysis Inhibitor (TAFI) as designed by McMurray et al.85 TAFI inhibits clot degradation and has been implicated in thrombotic disease. In the presence of TAFI the three lysine residues in the contrast agent were sequentially cleaved to a diphenylalanine or a 3,5-diiodotyrosine group, and both favor a strong binding ability to HSA and enhance the relaxivity of the contrast agent consequently (Fig. 10). The relaxivities of TAFI-induced RIME have increased to over 100% depending on the resulting diphenylalanine or 3,5-diiodotyrosine groups after cleavage. By using the RIME approach, Nagano et al. designed a β-galactosidase-activated MRI contrast agent.86 In the presence of β-galactosidase, the galactopyranose residue in the complex [Gd-5] (Gd-DTPA conjugate with albumin binding moiety that is masked by the galactopyranose residue) is cleaved from the aryl group by β-galactosidase and transformed to [Gd-8] (Gd-DTPA conjugate with albumin binding moiety), leading to an increase of the r1 relaxivity in phosphate-buffered saline with HSA due to favorable HSA binding (Fig. 11). Efficient polymerization of Gd3+ complexes can also be used to test the activity of enzymes such as myeloperoxidase (MPO). In the presence of enzyme and hydrogen peroxide, the oxidation of phenolic substrates generates free radicals, which polymerize to the oligomeric Gd3+ complex. Recently, two novel DTPA-bisamide derivatives bearing tyramido or 5-hydroxytryptamido groups were designed as enzyme-activated contrast agents for MRI, as reported by Bogdanov et al.87 The relaxivity values of monomers 1Gd3+ and 2Gd3+ (Fig. 12, top) are 4.6 and 4.5 mM−1 s−1 in deionized water (pH 4.8), and 4.3 and 4.3 mM−1 s−1 (0.47 T, 40 °C) under physiological conditions (10 mM sodium phosphate, 0.15 M NaCl, pH 7.4) respectively. After oligomerization by incubating the desired monomer solution with an excess of 3% H2O2 and Horseradish peroxidase for 1 h at 40 °C (Fig. 12, bottom), the relaxivities of monomers 1Gd3+ and 2Gd3+ are 15.9 and 10.5 respectively. By myeloperoxidase-mediated activation, the 5-hydroxytryptamide moiety of bis-5-hydroxytryptamide-GdDTPA (MPO-Gd) is oxidized and radicalized, forms a polymer with increased relaxivity, and could lead to in vivo assessment of myeloperoxidase activity in injured myocardium88 and tracking the inflammatory response in stroke in vivo.89
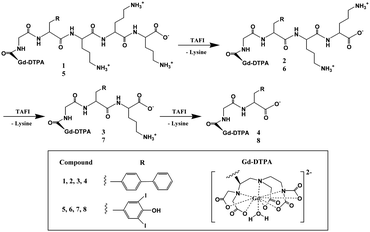 | ||
| Fig. 10 Bioactivated Gd3+ contrast agents: a Gd3+ chelate is coupled to an HSA binding moiety that is masked by an HSA shield group. Enzyme activation releases the shielding group and promotes HSA binding (adapted from ref. 85). | ||
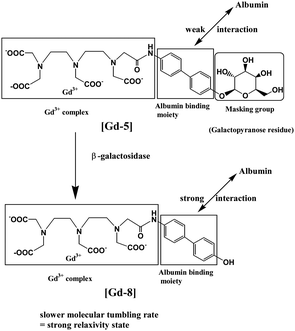 | ||
| Fig. 11 A mechanism of enhancement of relaxivity (adapted from ref. 86). | ||
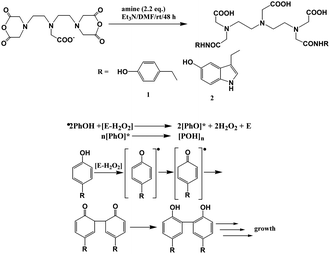 | ||
| Fig. 12 (Top) Synthesis and structures of compounds 1 and 2. (Bottom) Scheme of oxidoreductase-mediated reaction of phenol oligomerization (adapted from ref. 87). | ||
A general strategy for developing enzyme-responsive contrast agents is to conjugate a masking group which is relatively MR-silent, such as by having low HSA affinity or water access blocked by enzyme substrates. The masking group will be cleaved to render higher relaxivity in the presence of a specific enzyme. A significant change of relaxivity is needed to acquire an MR image at the molecular level. The design of such a system has been proven successful on the above mentioned examples. However, when the system was applied in vivo, anions and some organic molecules in live specimens can potentially affect the coordination environment of Gd(III) and interfere with the activation mechanism. For example, for Gd(III) chelates with seven coordinate macrocyclic ligands, such as cyclen possessing two inner-sphere water molecules, the expected high relaxivity caused by two inner-sphere waters is not verified in vivo as endogenous serum anions such as hydrogencarbonate or phosphate may replace the inner-sphere waters.84
D. Other small biomolecule-activated contrast agents
Compared to metal ion-responsive or enzyme-responsive smart contrast agents, only a few smart contrast agents are targeted for small biomolecules.90–93 As small biomolecules such as adenosine,90 glucose,91 lactate,92 and nitric oxide93 play important roles in biological systems, developing small biomolecule-activated contrast agents is one of the future directions. A general strategy for developing a smart MRI contrast agent for the sensing of small molecules based on DNA aptamers is reported by Lu and coworker.90 The system consists of an adenosine aptamer strand with a protein streptavidin, which hybridized a Gd-strand in which an amine group is conjugated to GdDOTA (Fig. 13). In the presence of adenosine, the Gd-strand will dissociate from the aptamer–streptavidin conjugate, leading to a 30% r1 relaxivity decrease mainly due to the lower molecular weight of the dissociated Gd-strand.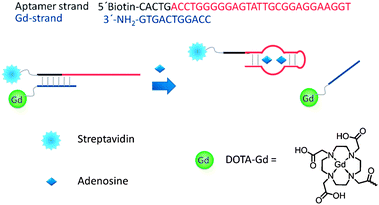 | ||
| Fig. 13 Scheme of the design of the adenosine-responsive MRI contrast agent based on DNA aptamer (reproduced from ref. 90 with permission from the Royal Society of Chemistry). | ||
Reversible responsive PARACEST MRI contrast agents have been designed that change their chemical exchange rates after binding to glucose,91 or that change the chemical shift of the amide protons after binding to lactate.92 PARACEST MRI contrast agents, Ln(III)-DO3A-orthoaminoanilide (Ln-DO3A-oAA, Ln = Yb, Gd), were developed by Pagel et al. to detect nitric oxide.93 In the presence of oxygen, the nitric oxide converts to N-nitroso intermediate by the endogeneous autooxidation. The aromatic amines of the contrast agent Yb(1) react with the N-nitroso intermediate, forming a triazene on Yb(2) and leading to an irreversible covalent change (Fig. 14). The strategy provides a potential application for in vivo irreversible responsive PARACEST MRI contrast agents.
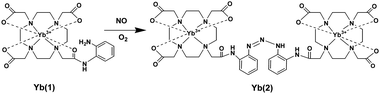 | ||
| Fig. 14 Illustration of the reaction of Yb(1) with NO in the presence of oxygen that converts aromatic amines to a triazene on Yb(2) (adapted from ref. 93). | ||
4. Blood pool contrast agents
Contrast-enhanced MRI is important in the detection, diagnosis, and staging of many types of cancer,96–102 as well as in the assessment of treatment response. Major oncology goals with MRI include imaging of tumor microvasculature characteristics and grading of tumor angiogenesis and neovasculture.103,104 MR imaging approaches to angiogenesis have considerable potential, which would be enhanced by contrast agents with multiplied relaxivity.105–108 Dynamic contrast-enhanced MRI combined with multiplied contrast relaxivity could lead to the improved resolution needed to meet these goals.109Blood pool contrast agents have been developed by several approaches to target the three components of blood vessels: plasma proteins, cells and water.110 In order to efficiently image blood vessels, macromolecular agents and targeting agents need to stay in the circulation and should not redistribute in the interstitial space.111 Technical requirements, protocol optimization and other applications of magnetic resonance angiography using blood pool contrast agents have been discussed in a recent review.112
A. Non-covalent binding to plasma proteins
The albumin-binding Gd(III) chelates are normally low molecular weight blood pool contrast agents. These compounds are able to bind non-covalently and reversibly to plasma albumin and will be completely excreted through the kidneys or via the hepatobiliary route. They overcame the macromolecular agents' disadvantages of not being completely excreted resulting in higher toxicity. Gadofosveset trisodium (MS-325 or Vasovist),113–117 Gd-EOB-DTPA (gadolinium 3,6,9-tris(carboxymethyl-4-(4-ethoxybenzyl)-undecandicarboxylic acid))118–120 and B22956 (gadocoletic acid trisodium salt)121,122 (Fig. 15) are the representative contrast agents of non-covalent binding to plasma. These Gd(III) chelates binding to human serum albumin (HSA) will prolong plasma half-life, retain the agent in the blood pool, and increase the relaxation rate of water protons in plasma. The current and future perspectives of MS-325 have been addressed.123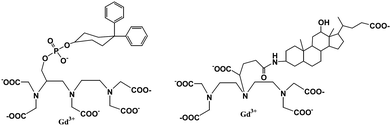 | ||
| Fig. 15 Chemical structures of MS-325 (left) and B22956 (right) (adapted from ref. 113 and 121). | ||
A series of Gd(III) complexes containing amphiphilic ligands with one or more hydrophobic moieties attached to the chelator which balance the lipophilicity and hydrophilicity of the compound have been synthesized.124–126 Suzuki et al. recently reported the design of six novel sulfonated contrast agent candidates (KMR-Sufol1 to KMR-Sufol6) for blood pool contrast agents (Fig. 16).127 The lipophilicity and the hydrophilicity of the Gd(III) complexes were investigated in order to increase the plasma half-life by binding to human serum albumin. The results showed that KMR-Sulfo5 has a relaxivity r1 of 5.9 mM−1 s−1 and a long plasma half-life of 25.7 min. And it was completely excreted from the body within 12 h after the administration. KMR-Sulfo5 has the potential to act as a blood pool contrast agent.
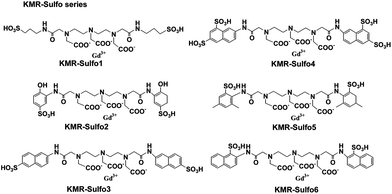 | ||
| Fig. 16 Structures of KMR-Sulfol-6 (adapted from ref. 127). | ||
We have developed two MRI contrast agents (CAs) comprised of Gd-DO3A conjugated with amino acid building blocks derived from glutamic acid (CA1) and lysine (CA2) (Fig. 17).128 The longitudinal relaxivities (r1) of two CAs measured at 9.4 T are 6.4 and 5.4 mM−1 s−1 in H2O at 25 °C for CA1 and CA2, respectively. Both longitudinal relaxivities (r1) are higher than that of clinically used Gd-DTPA (Magnevist, Bayer Schering, Germany) and Gd-DOTA (Dotarem, Guerbet, France). In vivo imaging in Wistar rats demonstrated considerable signal enhancement in the brain artery for CA2, but lower signal enhancement for CA1. Compared with Dotarem, which showed a similar signal enhancement as CA2, the enhancement by CA2 remained high even at 52 min post-injection. The long blood half-life (68.1 min) of CA2 indicated its binding to human serum albumin.
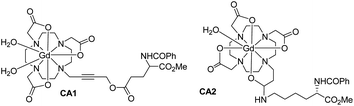 | ||
| Fig. 17 Chemical structures of CA1 and CA2 (adapted from ref. 128). | ||
B. Polymeric contrast agents
Some serum albumin–Gd(III) chelate conjugates have been developed as macromolecular agent prototypes in magnetic resonance angiography.129 But these agents have one or more of the following drawbacks: enhancement of interstitial space rather than blood pool only, immunogenicity of the complex, cardiac toxicity, prolonged retention of the complex in liver and bone. Since then, attention has been put on the development of macromolecular compounds as an alternative. Gd-DTPA poly(L-lysine) has been extensively evaluated as a macromolecular MR imaging contrast agent.130–132 Low molecular weight poly(L-lysine) has a rapid clearance whereas large molecular weight poly(L-lysine) has a longer blood half-life. However, a disadvantage of the DTPA-modified poly(L-lysine) is longer circulation time in kidneys and adrenal glands.Lu and coworkers reported a class of disulfide Gd(III) chelates as biodegradable macromolecular magnetic resonance imaging agents.133–139 The first polydisulfide agent, Gd-DTPA cystamine copolymer (GDCC), has been prepared and tested as an extracellular biodegradable blood pool contrast agent.131 GDCC was gradually degraded into smaller Gd(III) complex units by the cleavage of the disulfide bonds in the polymer backbone via the disulfide–thiol exchange reaction in the presence of 15 μM cysteine (Fig. 18). GDCC resulted in significant and prolonged contrast enhancement in the cardiovascular systems in rats, and was excreted rapidly via renal filtration.
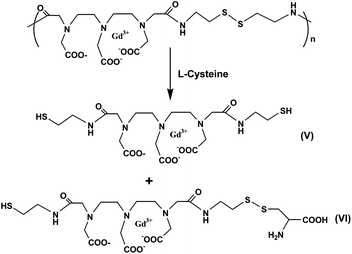 | ||
| Fig. 18 Degradation of Gd-DTPA cystamine copolymers (GDCC) in the presence of cysteine (adapted from ref. 133). | ||
Later, structures of the polydisulfides have been modified in order to improve the physicochemical properties, pharmaco-kinetics, and in vivo contrast enhancement of the biodegradable MRI contrast agents. Functional groups have been introduced around the disulfide bonds by replacing cystamine with cystine. In addition, different substituents have been attached to cystine (Fig. 19).134,135 The structural modification of polydisulfide Gd(III) complexes has resulted in biodegradable macromolecular contrast agents with various enhancement profiles in the blood pool. The agents are effective for cardiovascular and cancer MR imaging. They have a great potential to be developed as safe, effective, biodegradable macromolecular MRI contrast agents for clinical applications.
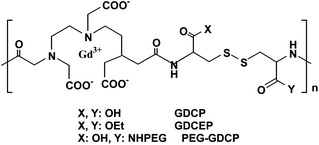 | ||
| Fig. 19 Structure of Gd-DTPA cystine copolymers (GDCP) and modified GDCP (PEG, poly(ethylene glycol); GDCEP, Gd-DTPA cystine diethyl ester copolymer) (adapted from ref. 134). | ||
Li et al. reported that the poly(L-glutamic acid) (PG) gadolinium chelates PG-Hex-DTPA-Gd and PG-Bz-DTPA-Gd (Fig. 20) were designed and prepared as biodegradable blood pool MRI contrast agents.140 The experiments showed that PG-Hex-DTPA-Gd was undegradable as 6-amino-hexyl side chains partially cross-linked with poly(L-glutamic acid) Gd-DTPA polymer; only linear PG-Bz-DTPA-Gd polymer was degradable in the presence of cathepsin B. A biodistribution study demonstrated that PG-Bz-DTPA-Gd was gradually cleared from the body and had significantly less retention in the blood, the spleen and the kidney. In vivo MRI in mice showed contrast enhancement in blood at up to 2 h post-injection of PG-Bz-DTPA-Gd.
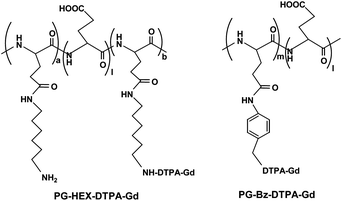 | ||
| Fig. 20 Structures of PG-Hex-DTPA-Gd and PG-Bz-DTPA-Gd (adapted from ref. 140). | ||
The same group recently reported micelles based on biodegradable poly(L-glutamic acid)-b-polylactide with Gd(III) chelated to the shell layer (Fig. 21) as a potential nanoscale MRI-visible delivery system.141 Nanoscale micelles of poly(L-glutamic acid)(DTPA-Gd)-b-polylactide copolymer exhibited almost two times higher T1-relaxivity than that of the low molecular-weight DTPA-Gd. Cho and coworkers prepared polysuccinimide (PSI) derivatives incorporating methoxy-poly(ethylene glycol) (mPEG) conjugated with DTPA-Gd as polymeric micelles (Fig. 22).142 The compounds have been tested as potential MRI contrast agents. In vitro MRI images of phantom showed better contrast compared to that of Omniscan. However, the pharmacokinetics of these polymeric micelles need to be determined before assessing their potential application in in vivo MRI.
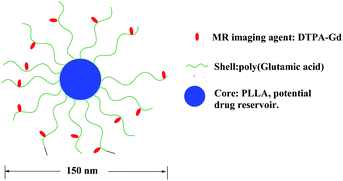 | ||
| Fig. 21 Schematic model of the micellar structure with DTPA-Gd chelated to the shell layer (adapted from ref. 141). | ||
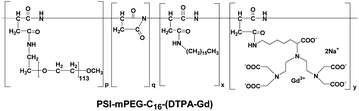 | ||
| Fig. 22 Structure of PSI-mPEG-C16-(DTPA-Gd) (adapted from ref. 142). | ||
C. Dendrimeric contrast agents
A class of dendrimer-based MRI contrast agents with large proton relaxation enhancements and high molecular relaxivities was reported by Wiener et al.143 These contrast agents, formed by the conjugation of 2-(4-isothiocyanatobenzyl)-6-methyl-diethylene-triamine-pentaacetic acid to the primary amines of ammonia core polyamidoamine (PAMAM) dendrimers, have high molecular relaxivities and greatly enhance the contrast of MR images when they are conjugated with Gd(III) chelates because the macromolecules slow down molecular rotation and decrease the molecular tumbling time of the gadolinium. Kobayashi and coworkers prepared and compared two core types of PAMAM dendrimer-based macromolecular MR contrast agents based on generation-6 polyamidoamine dendrimers (G6) (Fig. 23) according to their blood retention, tissue distribution, and renal excretion.144 The defined structure and large number of available surface amino groups of PAMAM dendrimers have led to their use as substrates in the preparation of novel MRI agents. MRI studies showed that the larger molecular weight contrast agent G6E-(1B4M-Gd)256, comprised of PAMAM dendrimers with an ethylenediamine core (G6E), 256 exterior primary amino groups and 2-(p-isothiocyanatobenzyl)-6-methyl-diethyl-enetriamine-pentacetic acid (1B4M) conjugated with Gd-DTPA, demonstrated longer blood retention and lower renal accumulation than that of G6A-(1B4M-Gd)192, comprised of PAMAM dendrimers with ammonia core (G6A), only 192 exterior primary amino groups and 2-(p-isothiocyanatobenzyl)-6-methyl-diethyl-enetriamin-epentacetic acid (1B4M) conjugated with Gd-DTPA. In terms of the ability of intravascular contrast agents, G6E-(1B4M-Gd)256 is better than G6A-(1B4M-Gd)192 due to more Gd(III) atoms and long retention time in the circulation.144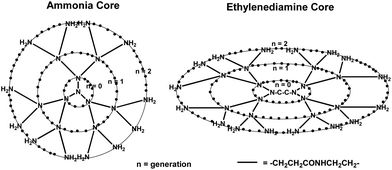 | ||
| Fig. 23 Scheme of the two core types of PAMAM dendrimers (adapted from ref. 144). | ||
Kobayashi and Brechbiel further developed one new core dendrimer, polypropylenimine diaminobutane (DAB) (Fig. 24), and reviewed the nano-sized MRI contrast agents with different dendrimer cores.145 These dendrimer-based macromolecular MRI contrast agents of various sizes and properties prepared are readily available and can provide sufficient contrast enhancement for various applications.146–148 Molecules up to 20 nm in diameter behave differently in the body depending on their size. Changes in molecular size up to 15 nm in diameter altered permeability across the vascular wall, excretion route, and recognition by the reticuloendothelial system.145 The in vivo and in vitro assessment demonstrated that Gd(III) chelates' assembly of dendrimers is much more efficient in modulating and relaxing water protons compared to a single chelate unit and could be used as intravascular contrast-enhancing agent.149
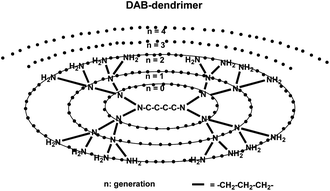 | ||
| Fig. 24 Scheme of the dendrimer core used for contrast agents (adapted from ref. 145). | ||
The use of Gd(III) chelates conjugated to high molecular weight dendrimers such as PAMAM prolongs intravascular retention and circulation time; slowing down molecular rotation will result in a shorter relaxation time and an increase in relaxivity as well. Such high relaxivities would increase the sensitivity of MRI and enable new types of diagnostic and targeted imaging. It is worth to review the high relaxivity dendrimeric Gd(III) chelates, although these agents are not within the scope of blood pool agents. Raymond et al. reported a new dendrimeric derivative of a hydroxypyridonated-based Gd(III) chelate with fast water exchange and high relaxivity at high magnetic field strength.150 This high relaxivity is partially because the two water molecules coordinated to the Gd(III) ion in each Gd(III) chelate. The alcohols of the dendrimer increase the water access to the complex, and the compactness of the whole molecule increases the rotational correlation time. Both of these contributed to the high relaxivity of the dendrimer at a high magnetic field. The same group recently developed a degradable dendrimer by employing esteramide and branched poly-L-lysine having up to eight gadolinium complexes per dendrimer.151 The relaxivity of each Gd base is much higher than that of one Gd(III) small molecule complex. Tei and coworkers reported that Gd(III) with heptadentate ligand AAZTA (6-amino-6-methylperhydro-1,4-diaepinetetraacetic acid) was attached to a polyamine or PAMAM G1 dendrimer forming octameric Gd(III) chelates.152 The relaxivity of each Gd of dendrimeric Gd(III) chelates is much higher than that of a single Gd(III) low molecular weight contrast agent. The two water molecules coordinated Gd(III) in each unit, the compact and rigid structures of dendrimers contribute to the high relaxivity of the contrast agent. Sherry et al. reported a pH-responsive GdDOTA-4AmP5− Gd(III) chelate35 to attach to a PAMAM G5 dendrimer to form a dendrimeric based pH-responsive contrast agent.153 As there are 96 Gd(III) chelates per dendrimer, the relaxivity of the dendrimer showed a doubled pH response from pH 9 to pH 6 per Gd compared to its single Gd(III) chelate.
Dendrimers have been used for preparing multifunctional macromolecular nanomaterials for diagnostic and therapeutic agents.154 Gd(III) chelates conjugated to dendrimers with functional groups are applicable to targeting contrast agents, and could be used as carriers for site-specific delivery of drugs without changing the property of the molecule attached. Tsourkas and coworkers recently reported novel gadolinium conjugated dendrimer nanoclusters as a tumor-targeted T1 MRI contrast agent.155 The dendrimer nanoclusters (DNCs) were fabricated by crosslinking G5 PAMAM dendrimers using a bifunctional amine-reactive crosslinker NHS-(PEG)5–NHS-(BS(PEG)5). Following DNC formation, paramagnetic Gd3+ ions were conjugated to DNCs by DTPA. The resulting paramagnetic DNCs were further functionalized with the tumor-targeting ligand folic acid and the optical imaging dye fluorescein isothiocyanate (FITC) (Fig. 25). The r1 relaxivity of gadolinium-conjugated DNCs is 12.3 mM−1 s−1 (1.41 T at 40 °C) per Gd and higher than that of Gd-DTPA. In vivo studies in folate-positive KB tumor mice showed that contrast enhancement increased significantly at 4 h to 24 h post-intravenous injection of DNCs.
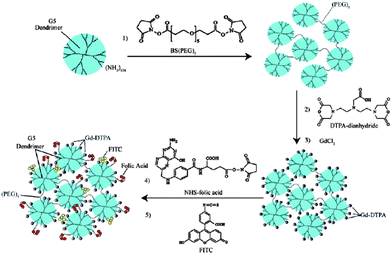 | ||
| Fig. 25 The preparation of paramagnetic targeted dendrimer nanoclusters (DNCs) (from ref. 155, copyright Wiley-VCH Verlag GmbH & Co. KGaA. Reproduced with permission). | ||
5. Blood–brain barrier permeable contrast agents
Delivery of drug, diagnostic or therapeutic proteins across the blood–brain barrier (BBB) can be achieved by invasive direct injection including permeabilization of tight junctions using either osmotic disruption by mannitol or biochemical opening, by a pharmacological approach, or by physiological approaches such as transport-mediated delivery and receptor-mediated transcytosis.156,157 In order to image structure and function in the brain to provide the physiological and pathophysiological information, several molecular imaging modalities such as MRI, optical, and PET have been developed for research and clinical applications. However, the development has been restrained by the ability to deliver imaging probes, especially contrast agents, across the BBB. Chemical compounds such as benzothiazoles, stilbenes, and imidazopyridines have been developed as biomarkers for Alzheimer's disease (AD) imaging.158–160 [11C]PIB160,161 (Pittsburgh Compound B, a thioflavin-T derivative), aminonaphthalene derivative [18F] FDDNP162 (2-(1-[6[(2-[18F]-fluoroethyl)(methyl)amino]-2-naphthyl]ethylidene)malono nitrile) and [11C]SB-13163 (a stilbene derivative) have been investigated for amyloid radioligands to image amyloid in patients with AD.Most of the Gd(III) chelate contrast agents used for brain tumor imaging are based on the diffusion through the leaky BBB under pathological conditions associated with the degradation of the blood–brain barrier such as P792 (Gadomelitol, Vistarem®), a high molecular weight gadolinium-based macromolecular intravascular contrast agent.164,165 Gd-DOTA was reported to characterize tumor angiogenesis using MRI in rats' brain.166 With mannitol to transiently open the BBB, intra-arterial injection of Gd-DTPA conjugated Aβ1-40 peptide could detect Aβ plaques in the brains of transgenic mice.167
Spires-Jones et al.168 developed a biodegradable nanocarrier system to deliver BBB-impermeable molecule imaging probes including staining reagents for multiphoton microscopy and Gd-based MRI contrast agents into the brain for neuroimaging. The nanocarrier system is composed of poly(n-butyl cyanoacrylate) dextran polymers coated with polysorbate 80 (PBCA nanoparticles) which absorb plasma apolipoprotein E through vascular endothelial cells by receptor-mediated transcytosis to cross the BBB without disruption. Low-intensity lipoprotein receptor-related protein-1 (LRP-1) can be used for delivery of therapeutics from blood to the brain such as Angiopep-2 by receptor-mediated transcytosis.169 It indicated that LRP1 not only expresses at the abluminal side but expresses at the luminal side as well. LRP1 has a significant role in regulating brain and systemic clearance of Alzheimer's Aβ.170 Development of LRP1-based therapies for AD may provide the approach for delivery of Gd-based contrast agents that cross the BBB by receptor-mediated transcytosis.
Poduslo and coworkers designed and synthesized a contrast agent Gd[N-4ab/Q-4ab]Aβ30 to cross the BBB by means of a receptor-mediated transport system.171 It is a derivative of human amyloid-β (Aβ) peptide based on the sequence of the first 30 amino acid residues of Aβ with asparagyl/blutamyl-4-aminobutane residues (N-4AB/Q4ab) substituted at unique Asp and Glu positions and with Gd-DTPA-aminohexanoic acid covalently attached to the N-terminal Asp (Fig. 26). The chemical modification reaction was employed to modify polyamine proteins to target carboxyl groups of aspartic and glutamic acid residues using water soluble carbodiimide. The contrast agent Gd[N-4ab/Q-4ab]Aβ30 demonstrated enhanced in vitro binding to Alzheimer's disease amyloid plaques and high BBB permeability in vivo in Alzheimer's disease transgenic mouse brain. The pharmacokinetics and amyloid plaque targeting ability of the contrast agents were investigated in Alzheimer's disease transgenic mice.172
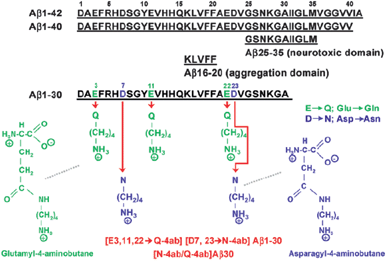 | ||
| Fig. 26 Molecular structure of diamine-substituted Aβ1-30. Amino acid sequence of Aβ peptides and chemical structure of modified glutamine and asparagine residues (reproduced with permission from ref. 171. Copyright (2012) American Chemical Society). | ||
Using 125I in radioiodination, the 125I-Gd[N-4ab/Q-4ab]Aβ30 contrast agent was injected via intravenous bolus administration; it demonstrated rapid nonsaturable absorption at the BBB. The brain pharmacokinetics showed a rapid absorption phase followed by a slower elimination phase. Emulsion autoradiography studies showed plaque specific accumulation of the contrast agent in the brain for an extended period of time. The same group also reported a polyamine modified pF(ab′)2 4.1 antibody fragment of a monoclonal antibody, Immunoglobulin (IgG) 4.1, raised against the fibrillar human amyloid protein Aβ42, which showed increased BBB permeability with retained antigen binding ability to Aβ peptides and amyloid plaques under in vitro conditions.173 Conjugation of Gd-DOTA to radioiodinated pF(ab′)2 4.1 (125I-Gd-DOTA-pF(ab′)2 4.1) makes the contrast agent plaque specific and having high BBB permeability and low accumulation in the liver and kidney.174
6. Conclusions and future outlook
With insight into physico-chemical parameters and structure-related relaxation mechanisms, the design of monomeric Gd(III) chelates as small molecule contrast agents is a mature field. Most Gd-based contrast agents for preclinical and clinical applications nowadays are monomeric Gd(III) chelates. Knowledge of specific physiological or biochemical processes such as changes in pH, metal ions, enzymatic activity, and small biomolecules allows researchers to better design smart probes for different and specific purposes. For smart MRI contrast agents and blood pool agents, the challenge is to apply these agents for in vivo study and their toxic effect needs to be considered. A lot of opportunities are available for making BBB permeable: contrast agents and other specific targeting agents such as tumor targeting and tissue targeting agents.Since high relaxivities would enable new types of diagnostic and targeted imaging, with instrumental resolution advances and further development of dynamic MRI, imaging of changes in angiogenesis and monitoring anti-angiogenic therapies would be possible. Gd(III) chelates could be incorporated into novel targeted MRI contrast agents by covalent attachment to targeting moieties such as RGD peptides via bioconjugation strategies, and new classes of imaging challenges can be addressed. One of the major challenges in this field is the non-invasive assessment of vector delivery and transgene expression.175 One strategy for monitoring viral vector delivery by MRI would be to bioconjugate Gd(III) chelates to the vector. The chemical coupling would need to be done in a manner that does not interfere with efficient gene delivery. Strategies for monitoring transgene expression depend on the nature and localization of the transgene product.
Acknowledgements
This work was supported by the Singapore Bioimaging Consortium Intramural Funding of the Biomedical Research Council; COT Grant ETPL/10-S10COT-006; JCO Grant 1131CFG002 of A*STAR (Agency for Science, Technology and Research), Singapore. The authors are grateful to Dr. Edward G. Robins, Professor David W. Townsend and Professor Philip W. Kuchel for their helpful comments. The authors also thank the reviewers’ for their invaluable comments that help improve the quality of this review article.Notes and references
- W. Schima, A. Mukerjee and S. Saini, Clin. Radiol., 1996, 51, 235–244 CrossRef CAS.
- P. Caravan, J. J. Ellison, T. J. McMurry and R. B. Lauffer, Chem. Rev., 1999, 99, 2293–2352 CrossRef CAS.
- S. Aime, A. Barge, C. Cabella, S. G. Crich and E. Gianolio, Curr. Pharm. Biotechnol., 2004, 5, 509–518 CAS.
- K. W.-Y. Chan and W.-T. Wong, Coord. Chem. Rev., 2006, 250, 1562 CrossRef.
- Textbook of Contrast Media, ed. P. Dawson, D. O. Cosgrove and R. G. Grainger, Isis Medical Media Ltd, Oxford, UK, 1999, section II Search PubMed.
- S. C. Jackels, Enhancement Agents for Magnetic Resonance Imaging: Fundamentals, in Pharmaceuticals in Medical Imaging, ed. D. P. Swanson, H. M. Chilton and J. H. Thrall, Macmillan, New York, 1990, p. 655 Search PubMed.
- R. Mathur-De Vré and M. Lemort, Br. J. Radiol., 1995, 68, 225–247 CrossRef.
- P. Hermann, J. Kotek, V. Kubíček and I. Lukeš, Dalton Trans., 2008, 3027–3047 RSC.
- J. A. Peters, J. Huskens and D. J. Raber, Prog. Nucl. Magn. Reson. Spectrosc., 1996, 28, 283–350 CrossRef CAS.
- S. H. Koenig and R. D. Brown, III, Prog. Nucl. Magn. Reson. Spectrosc., 1990, 22, 487–567 CrossRef CAS.
- S. Aime, M. Botta, M. Fasano and E. Terreno, Chem. Soc. Rev., 1998, 27, 19–29 RSC.
- S. M. Cohen, J. Xu, E. Radkov, K. N. Raymond, M. Botta, A. Barge and S. Aime, Inorg. Chem., 2000, 39, 5747–5756 CrossRef CAS.
- Z. Jászberényi, L. Moriggi, P. Schmidt, C. Weidensteiner, R. Kneuer, A. E. Merbach, L. Helm and É. Tóth, JBIC, J. Biol. Inorg. Chem., 2007, 12, 406–420 CrossRef.
- S. Langereis, A. Dirksen, T. M. Hackeng, M. H. P. van Genderen and E. W. Meijer, New J. Chem., 2007, 31, 1152–1160 RSC.
- S. Laus, A. Sour, R. Ruloff, É. Tóth and A. E. Merbach, Chem.–Eur. J., 2005, 11, 3064–3076 CrossRef CAS.
- D. A. Fulton, M. O'Halloran, D. Parker, K. Senanayake, M. Botta and S. Aime, Chem. Commun., 2005, 474–476 RSC.
- S. Aime, E. Gianolio, D. Corpillio, C. Cavallotti, G. Palmisano, M. Sisti, G. B. Giovenzana and R. Pagliarin, Helv. Chim. Acta, 2003, 86, 615–632 CrossRef CAS.
- S. Aime, L. Calabi, C. Cavallotti, E. Gianolio, G. B. Giovenzana, P. Losi, A. Maiocchi, G. Palmisano and M. Sisti, Inorg. Chem., 2004, 43, 7588–7590 CrossRef CAS.
- A. R. Johnson, B. O'Sullivan and K. N. Raymond, Inorg. Chem., 2000, 39, 2652–2660 CrossRef CAS.
- E. J. Werner, S. Avedano, M. Botta, B. P. Hay, E. G. Moore, S. Aime and K. N. Raymond, J. Am. Chem. Soc., 2007, 129, 1870–1871 CrossRef CAS.
- R. S. Ranganathan, M. E. Fernandez, S. I. Kang, A. D. Nunn, P. C. Ratsep, K. M. R. Pillai, X. Zhang and M. F. Tweedle, Invest. Radiol., 1998, 33, 779–797 CrossRef CAS.
- V. C. Pierre, M. Botta, S. Aime and K. N. Raymond, J. Am. Chem. Soc., 2006, 128, 9272–9273 CrossRef CAS.
- J. B. Livramento, É. Tóth, A. Sour, A. Borel, A. E. Merbach and R. Ruloff, Angew. Chem., Int. Ed., 2005, 44, 1480–1484 CrossRef CAS.
- J. B. Livramento, A. Sour, A. Borel, A. E. Merbach and É. Tóth, Chem.–Eur. J., 2006, 12, 989–1003 CrossRef CAS.
- J. B. Livramento, L. Helm, A. Sour, C. O'Neil, A. E. Merbach and É. Tóth, Dalton Trans., 2008, 1195–1202 RSC.
- P. Caravan, Chem. Soc. Rev., 2006, 35, 512–523 RSC.
- J. Xu, S. J. Franklin, D. W. Whisenhunt, Jr and K. N. Raymond, J. Am. Chem. Soc., 1995, 117, 7245–7246 CrossRef CAS.
- J. L. Sessler, T. D. Mody, G. W. Hemmi, V. Lynch, S. W. Young and R. A. Miller, J. Am. Chem. Soc., 1993, 115, 10368–10369 CrossRef CAS.
- J. M. Bryson, W.-J. Chu, J.-H. Lee and T. M. Reineke, Bioconjugate Chem., 2008, 19, 1505–1509 CrossRef CAS.
- B. Jebasingh and V. Alexander, Inorg. Chem., 2005, 44, 9434–9443 CrossRef CAS.
- M. F. Tweedle, J. Alloys Compd., 1992, 180, 317–323 CrossRef CAS.
- R. B. Lauffer, Chem. Rev., 1987, 87, 901–927 CrossRef CAS.
- The Chemistry of Contrast Agents in Medical Magnetic Resonance Imaging, ed. A. E. Merbach and É. Tóth, John Wiley & Sons, Chichester, New York, 2001, p. 471 Search PubMed.
- P. Caravan, C. T. Farrar, L. Frullano and R. Uppal, Contrast Media Mol. Imaging, 2009, 4, 89–100 CrossRef CAS.
- S. Zhang, K. Wu and A. D. Sherry, Angew. Chem., Int. Ed., 1999, 38, 3192–3194 CrossRef CAS.
- F. K. Kálmán, M. Woods, P. Caravan, P. Jurek, M. Spiller, G. Tircsó, R. Király, E. Brücher and A. D. Sherry, Inorg. Chem., 2007, 46, 5260–5270 CrossRef.
- N. Raghunand, C. Howison, A. D. Sherry, S. Zhang and R. J. Gillies, Magn. Reson. Med., 2003, 49, 249–257 CrossRef CAS.
- M. L. Garcia-Martin, G. V. Martinez, N. Raghunand, A. D. Sherry, S. Zhang and R. J. Gillies, Magn. Reson. Med., 2006, 55, 309–315 CrossRef CAS.
- R. J. Gillies, N. Raghunand, M. L. Garcia-Martin and R. A. Gatenby, IEEE Eng. Med. Biol. Mag., 2004, 23, 57–64 CrossRef.
- M. Woods, S. Zhang, V. H. Ebron and A. D. Sherry, Chem.–Eur. J., 2003, 9, 4634–4640 CrossRef CAS.
- L. Frullano, C. Catana, T. Benner, A. D. Sherry and P. Caravan, Angew. Chem., Int. Ed., 2010, 49, 2382–2384 CrossRef CAS.
- S. Aime, S. G. Crich, M. Botta, G. Giovenzana, G. Palmisano and M. Sisti, Chem. Commun., 1999, 1577–1578 RSC.
- J. I. Bruce, R. S. Dickins, L. J. Govenlock, T. Gunnlaugsson, S. Lopinski, M. P. Lowe, D. Parker, R. D. Peacock, J. J. B. Perry, S. Aime and M. Botta, J. Am. Chem. Soc., 2000, 122, 9674–9684 CrossRef CAS.
- M. P. Lowe, D. Parker, O. Reany, S. Aime, M. Botta, G. Castellano, E. Gianolio and R. Pagliarin, J. Am. Chem. Soc., 2001, 123, 7601–7609 CrossRef CAS.
- K.-E. Løkling, S. L. Fossheim, R. Skurtveit, A. Bjørnerud and J. Klaveness, Magn. Reson. Imaging, 2001, 19, 731–738 CrossRef.
- K.-E. Løkling, R. Skurtveit, A. Bjørnerud and S. L. Fossheim, Magn. Reson. Med., 2004, 51, 688–696 CrossRef.
- K.-E. Løkling, S. L. Fossheim, J. Klaveness and R. Skurtveit, J. Controlled Release, 2004, 98, 87–95 CrossRef.
- N. Raghunand, C. Howison, A. D. Sherry, S. Zhang and R. J. Gillies, Magn. Reson. Med., 2003, 49, 249–257 CrossRef CAS.
- M. L. Garcia-Martin, G. V. Martinez, N. Raghunand, A. D. Sherry, S. Zhang and R. J. Gillies, Magn. Reson. Med., 2006, 55, 309–315 CrossRef CAS.
- K. B. Hartman, S. Laus, R. D. Bolskar, R. Muthupillai, L. Helm, É. Tóth, A. E. Merbach and L. J. Wilson, Nano Lett., 2008, 8, 415–418 CrossRef CAS.
- B. Sitharaman, R. D. Bolskar, I. Rusakova and L. J. Wilson, Nano Lett., 2004, 4, 2373–2378 CrossRef CAS.
- R. D. Bolskar, A. F. Benedetto, L. O. Husebo, R. E. Price, E. F. Jackson, S. Wallace, L. J. Wilson and J. M. Alford, J. Am. Chem. Soc., 2003, 125, 5471–5478 CrossRef CAS.
- É. Tóth, R. D. Bolskar, A. Borel, G. González, L. Helm, A. E. Merbach, B. Sitharaman and L. J. Wilson, J. Am. Chem. Soc., 2005, 127, 799–805 CrossRef.
- B. Sitharaman, L. A. Tran, Q. P. Pham, R. D. Bolskar, R. Muthupillai, S. D. Flamm, A. G. Mikos and L. J. Wilson, Contrast Media Mol. Imaging, 2007, 2, 139–146 CrossRef CAS.
- R. D. Bolskar, Nanomedicine, 2008, 3, 201–213 CrossRef CAS.
- H. Kato, Y. Kanazawa, M. Okumura, A. Taninaka, T. Yokawa and H. Shinohara, J. Am. Chem. Soc., 2003, 125, 4391–4397 CrossRef CAS.
- W. Li, G. Parigi, M. Fragai, C. Luchinat and T. J. Meade, Inorg. Chem., 2002, 41, 4018–4024 CrossRef CAS.
- W. Li, S. E. Fraser and T. J. Meade, J. Am. Chem. Soc., 1999, 121, 1413–1414 CrossRef CAS.
- E. L. Que and C. J. Chang, Chem. Soc. Rev., 2010, 39, 51–60 RSC.
- K. Dhingra, P. Fousková, G. Angelovski, M. E. Maier, N. K. Logothetis and É. Tóth, JBIC, J. Biol. Inorg. Chem., 2008, 13, 35–46 CrossRef CAS.
- A. Mishra, P. Fousková, G. Angelovski, E. Balogh, A. K. Mishra, N. K. Logothetis and É. Tóth, Inorg. Chem., 2008, 47, 1370–1381 CrossRef CAS.
- G. Angelovski, P. Fousková, I. Mamedov, S. Canals, É. Tóth and N. K. Logothetis, ChemBioChem, 2008, 9, 1729–1734 CrossRef CAS.
- K. Dhingra, M. E. Maier, M. Beyerlein, G. Angelovski and N. K. Logothetis, Chem. Commun., 2008, 3444–3446 RSC.
- H. Hifumi, A. Tanimoto, D. Citterio, H. Komatsu and K. Suzuki, Analyst, 2007, 132, 1153–1160 RSC.
- R. Trokowski, J. Ren, F. K. Kálmán and A. D. Sherry, Angew. Chem., Int. Ed., 2005, 44, 6920–6923 CrossRef CAS.
- A. C. Esqueda, J. A. López, G. Andreu-de-Riquer, J. C. Alvarado-Monzón, J. Ratnakar, A. J. M. Lubag, A. D. Sherry and L. M. De León-Rodríguez, J. Am. Chem. Soc., 2009, 131, 11387–11391 CrossRef CAS.
- K. Hanaoka, K. Kikuchi, Y. Urano and T. Nagano, J. Chem. Soc., Perkin Trans. 2, 2001, 1840–1843 RSC.
- K. Hanaoka, K. Kikuchi, Y. Urano, M. Narazaki, T. Yokawa, S. Sakamoto, K. Yamaguchi and T. Nagano, Chem. Biol., 2002, 9, 1027–1032 CrossRef CAS.
- J. L. Major, G. Parigi, C. Luchinat and T. J. Meade, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 13881–13886 CrossRef CAS.
- J. L. Major, R. M. Boiteau and T. J. Meade, Inorg. Chem., 2008, 47, 10788–10795 CrossRef CAS.
- X. Zhang, K. S. Lovejoy, A. Jasanoff and S. J. Lippard, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 10780–10785 CrossRef CAS.
- J. Paris, C. Gameiro, V. Humblet, P. K. Mohapatra, V. Jacques and J. F. Desreux, Inorg. Chem., 2006, 45, 5092–5102 CrossRef CAS.
- V. Comblin, D. Gilsoul, M. Hermann, V. Humblet, V. Jacques, M. Mesbahi, C. Sauvage and J. F. Desreux, Coord. Chem. Rev., 1999, 185–186, 451–470 CrossRef CAS.
- J. B. Livramento, É. Tóth, A. Sour, A. Borel, A. E. Merbach and R. Ruloff, Angew. Chem., Int. Ed., 2005, 44, 1480–1484 CrossRef CAS.
- T. N. Parac-Vogt, L. Vander Elst, K. Kimpe, S. Laurent, C. Burtéa, F. Chen, R. Van Deun, Y. Ni, R. N. Muller and K. Binnemans, Contrast Media Mol. Imaging, 2006, 1, 267–278 CrossRef CAS.
- R. Ruloff, G. van Koten and A. E. Merbach, Chem. Commun., 2004, 842–843 RSC.
- E. L. Que and C. J. Chang, J. Am. Chem. Soc., 2006, 128, 15942–15943 CrossRef CAS.
- E. L. Que, E. Gianolio, S. L. Baker, A. P. Wong, S. Aime and C. J. Chang, J. Am. Chem. Soc., 2009, 131, 8527–8536 CrossRef CAS.
- E. L. Que, E. Gianolio, S. L. Baker, A. P. Wong, S. Aime and C. J. Chang, Dalton Trans., 2010, 39, 469–476 RSC.
- R. A. Moats, S. E. Fraser and T. J. Meade, Angew. Chem., Int. Ed. Engl., 1997, 36, 726–728 CrossRef CAS.
- A. Y. Louie, M. M. Hüber, E. T. Ahrens, U. Rothbächer, R. Moats, R. E. Jacobs, S. E. Fraser and T. J. Meade, Nat. Biotechnol., 2000, 18, 321–325 CrossRef CAS.
- Y.-T. Chang, C.-M. Cheng, Y.-Z. Su, W.-T. Lee, J.-S. Hsu, G.-C. Liu, T.-L. Cheng and Y.-M. Wang, Bioconjugate Chem., 2007, 18, 1716–1727 CrossRef CAS.
- P. L. Anelli, I. Bertini, M. Fragai, L. Lattuada, C. Luchinat and G. Parigi, Eur. J. Inorg. Chem., 2000, 625–630 CrossRef CAS.
- M. Giardiello, M. P. Lowe and M. Botta, Chem. Commun., 2007, 4044–4046 RSC.
- A. L. Nivorozhkin, A. F. Kolodziej, P. Caravan, M. T. Greenfield, R. B. Lauffer and T. J. McMurry, Angew. Chem., Int. Ed., 2001, 40, 2903–2906 CrossRef CAS.
- K. Hanaoka, K. Kikuchi, T. Terai, T. Komatsu and T. A. Nagano, Chem.–Eur. J., 2008, 14, 987–995 CrossRef CAS.
- M. Querol, J. W. Chen, R. Weissleder and A. Bogdanov, Jr, Org. Lett., 2005, 7, 1719–1722 CrossRef CAS.
- M. Nahrendorf, D. Sosnovik, J. W. Chen, P. Panizzi, J.-L. Figueiredo, E. Aikawa, P. Libby, F. K. Swirski and R. Weissleder, Circulation, 2008, 117, 1153–1160 CrossRef CAS.
- M. O. Breckwoldt, J. W. Chen, L. Stangenberg, E. Aikawa, E. Rodriguez, S. Qiu, M. A. Moskowitz and R. Weissleder, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 18584–18589 CrossRef CAS.
- W. Xu and Y. Lu, Chem. Commun., 2011, 47, 4998–5000 RSC.
- S. Zhang, R. Trokowski and A. D. Sherry, J. Am. Chem. Soc., 2003, 125, 15288–15289 CrossRef CAS.
- S. Aime, D. Delli Castelli, F. Fedeli and E. Terreno, J. Am. Chem. Soc., 2002, 124, 9364–9365 CrossRef CAS.
- G. Liu, Y. Li and M. D. Pagel, Magn. Reson. Med., 2007, 58, 1249–1256 CrossRef CAS.
- V. Jacques and J. F. Desreux, Contrast Agents I. Magnetic Resonance Imaging, in Topics in Current Chemistry, ed. W. Krause, Springer-Verlag, Berlin, 2002, vol. 221, pp. 123–164 Search PubMed.
- M. P. Lowe, Curr. Pharm. Biotechnol., 2004, 5, 519–528 CAS.
- G. J. Strijkers, W. J. M. Mulder, G. A. F. van Tilborg and K. Nicolay, Anti-Cancer Agents Med. Chem., 2007, 7, 291–305 CrossRef CAS.
- C. F. G. C. Geraldes and S. Laurent, Contrast Media Mol. Imaging, 2009, 4, 1–23 CrossRef CAS.
- D. Akata, U. Kerimoglu, T. Hazirolan, M. Karcaaltincaba, F. Köse, M. N. Özmen and O. Akhan, Eur. Radiol., 2005, 15, 1727–1733 CrossRef.
- B. K. Park, C. K. Kim, B. Kim and G. Y. Kwon, Abdom. Imaging, 2007, 32, 515–518 CrossRef.
- J. Ricke, J. Sehouli, C. Hach, E. L. Hänninen, W. Lichtenegger and R. Felix, Eur. Radiol., 2003, 13, 943–949 CrossRef.
- S. G. Orel and M. D. Schnall, Radiology, 2001, 220, 13–30 CAS.
- K. Turetschek, E. Floyd, T. Helbich, T. P. Roberts, D. M. Shames, M. F. Wendland, W. O. Carter and R. C. Brasch, J. Magn. Reson. Imaging, 2001, 14, 237–242 CrossRef CAS.
- M. Neeman and H. Dafni, Annu. Rev. Biomed. Eng., 2003, 5, 29–56 CrossRef CAS.
- M. Schirner, A. Menrad, A. Stephens, T. Frenzel, P. Hauff and K. Licha, Ann. N. Y. Acad. Sci., 2004, 1014, 67–75 CrossRef CAS.
- R. J. Gillies, N. Raghunand, G. S. Karczmar and Z. M. Bhujwalla, J. Magn. Reson. Imaging, 2002, 16, 430–450 CrossRef.
- B. D. Ross, T. L. Chenevert and A. Rehemtulla, Eur. J. Cancer, 2002, 38, 2147–2156 CrossRef CAS.
- A. R. Padhani, J. Magn. Reson. Imaging, 2002, 16, 407–422 CrossRef.
- M. Neeman, J. Cell. Biochem., 2002, 39, 11–17 CrossRef.
- M. V. Knopp, F. L. Giesel, H. Marcos, H. von Tengg-Kobligk and P. Choyke, Top. Magn. Reson. Imaging, 2001, 12, 301–308 CrossRef CAS.
- A. A. Bogdanov, Jr, M. Lewin and R. Weissleder, Adv. Drug Delivery Rev., 1999, 37, 279–293 CrossRef.
- V. V. Martin, W. H. Ralston, M. R. Hynes and J. F. W. Keana, Bioconjugate Chem., 1995, 6, 616–623 CrossRef CAS.
- M. Rohrer, L. Geerts-Ossevoort and G. Laub, Eur. Radiol., 2007, 17(2), B7–12 Search PubMed.
- D. J. Parmelee, R. C. Walovitch, H. S. Ouellet and R. B. Lauffer, Invest. Radiol., 1997, 32, 741–747 CrossRef CAS.
- R. B. Lauffer, D. J. Parmelee, S. U. Dunham, H. S. Ouellet, R. P. Dolan, S. Witte, T. J. McMurry and R. C. Walovitch, Radiology, 1998, 207, 529–538 CAS.
- T. M. Grist, F. R. Korosec, D. C. Peters, S. Witte, R. C. Walovitch, R. P. Dolan, W. E. Bridson, E. K. Yucel and C. A. Mistretta, Radiology, 1998, 207, 539–544 CAS.
- P. Caravan, N. J. Cloutier, M. T. Greenfield, S. A. McDermid, S. U. Dunham, J. W. M. Bulte, J. C. Amedio, Jr, R. J. Looby, R. M. Supkowski, W. D. Horrocks, Jr, T. J. McMurry and R. B. Lauffer, J. Am. Chem. Soc., 2002, 124, 3152–3162 CrossRef CAS.
- J. F. Meaney and M. Goyen, Eur. Radiol., 2007, 17(2), B2–B6 Search PubMed.
- L. Vander Elst, F. Maton, S. Laurent, F. Seghi, F. Chapelle and R. N. Muller, Magn. Reson. Med., 1997, 38, 604–614 CrossRef CAS.
- L. Vander Elst, F. Chapelle, S. Laurent and R. N. Muller, JBIC, J. Biol. Inorg. Chem., 2001, 6, 196–200 CrossRef CAS.
- H.-J. Weinmann, G. Schuhmann-Giampieri, H. Schmitt-Willich, H. Vogler, T. Frenzel and H. Gries, Magn. Reson. Med., 1991, 22, 233–237 CrossRef CAS.
- A. Preda, M. van Vliet, G. P. Krestin, R. C. Brasch and C. F. van Dijke, Invest. Radiol., 2006, 41, 325–331 CrossRef.
- C. de Haën, P. L. Anelli, V. Lorusso, A. Morisetti, F. Maggioni, J. Zheng, F. Uggeri and F. M. Cavagna, Invest. Radiol., 2006, 41, 279–291 CrossRef.
- C. Fink, M. Goyen and J. Lotz, Eur. Radiol., 2007, 17(2), B38–B44 Search PubMed.
- S. Aime, M. Botta, M. Fasano, S. G. Crich and E. Terreno, JBIC, J. Biol. Inorg. Chem., 1996, 1, 312–319 CrossRef CAS.
- S. Langereis, Q. G. de Lussanet, M. H. P. van Genderen, E. W. Meijer, R. G. H. Beets-Tan, A. W. Griffioen, J. M. A. van Engelshoven and W. H. Backes, NMR Biomed., 2006, 19, 133–141 CrossRef CAS.
- É. Tóth, F. Connac, L. Helm, K. Adzamli and A. E. Merbach, JBIC, J. Biol. Inorg. Chem., 1998, 3, 606–613 CrossRef.
- H. Hifumi, A. Tanimoto, A. Honda, D. Citterio and K. Suzuki, Anal. Sci., 2007, 23, 1159–1165 CrossRef CAS.
- K. Kittigowittana, C.-T. Yang, W. C. Cheah, K.-H. Chuang, C.-Y. Tuang, Y.-T. Chang, X. Golay and R. W. Bates, ChemMedChem, 2011, 6, 781–787 CrossRef CAS.
- R. B. Lauffer and T. J. Brady, Magn. Reson. Imaging, 1985, 3, 11–16 CrossRef CAS.
- G. Schuhmann-Giampieri, H. Schmitt-Willich, T. Frenzel, W. R. Press and H. J. Weinmann, Invest. Radiol., 1991, 26, 969–974 CrossRef CAS.
- V. S. Vexler, O. Clément, H. Schmitt-Willich and R. C. Brasch, J. Magn. Reson. Imaging, 1994, 4, 381–388 CrossRef CAS.
- A. A. Bogdanov, Jr, A. Alexei, R. Weissleder and T. J. Brady, Adv. Drug Delivery Rev., 1995, 16, 335–348 CrossRef.
- Z.-R. Lu, D. L. Parker, K. C. Goodrich, X. Wang, J. G. Dalle and H. R. Buswell, Magn. Reson. Med., 2004, 51, 27–34 CrossRef CAS.
- A. M. Mohs, X. Wang, K. C. Goodrich, Y. Zong, D. L. Parker and Z.-R. Lu, Bioconjugate Chem., 2004, 15, 1424–1430 CrossRef CAS.
- T. L. Kaneshiro, T. Ke, E.-K. Jeong, D. L. Parker and Z.-R. Lu, Pharm. Res., 2006, 23, 1285–1294 CrossRef CAS.
- Y. Zong, X. Wang, K. C. Goodrich, A. M. Mohs, D. L. Parker and Z.-R. Lu, Magn. Reson. Med., 2005, 53, 835–842 CrossRef CAS.
- T. Ke, Y. Feng, J. Guo, D. L. Parker and Z.-R. Lu, Magn. Reson. Imaging, 2006, 24, 931–940 CrossRef CAS.
- Z.-R. Lu, A. M. Mohs, Y. Zong and Y. Feng, Int. J. Nanomed., 2006, 1, 31–40 CrossRef CAS.
- A. M. Mohs, T. Nguyen, E.-K. Jeong, Y. Feng, L. Emerson, Y. Zong, D. L. Parker and Z.-R. Lu, Magn. Reson. Med., 2007, 58, 110–118 CrossRef CAS.
- X. Wen, E. F. Jackson, R. E. Price, E. E. Kim, Q. Wu, S. Wallace, C. Charnsangavej, J. G. Gelovani and C. Li, Bioconjugate Chem., 2004, 15, 1408–1415 CrossRef CAS.
- G. Zhang, R. Zhang, X. Wen, L. Li and C. Li, Biomacromolecules, 2008, 9, 36–42 CrossRef CAS.
- H. Y. Lee, H. W. Jee, S. M. Seo, B. K. Kwak, G. Khang and S. H. Cho, Bioconjugate Chem., 2006, 17, 700–706 CrossRef CAS.
- E. C. Wiener, M. W. Brechbiel, H. Brothers, R. L. Magin, O. A. Gansow, D. A. Tomalia and P. C. Lauterbur, Magn. Reson. Med., 1994, 31, 1–8 CrossRef CAS.
- H. Kobayashi, N. Sato, S. Kawamoto, T. Saga, A. Hiraga, T. L. Haque, T. Ishimori, J. Konishi, K. Togashi and M. W. Brechbiel, Bioconjugate Chem., 2001, 12, 100–107 CrossRef CAS.
- H. Kobayashi and M. W. Brechbiel, Adv. Drug Delivery Rev., 2005, 57, 2271–2286 CrossRef CAS.
- K. Nwe, H. Xu, C. A. S. Regino, M. Bernardo, L. Ileva, L. Riffle, K. J. Wong and M. W. Brechbiel, Bioconjugate Chem., 2009, 20, 1412–1418 CrossRef CAS.
- K. Nwe, L. H. Bryant, Jr and M. W. Brechbiel, Bioconjugate Chem., 2010, 21, 1014–1017 CrossRef CAS.
- K. Nwe, M. Bernardo, C. A. S. Regino, M. Williams and M. W. Brechbiel, Bioorg. Med. Chem., 2010, 18, 5925–5931 CrossRef CAS.
- K. Nwe, D. Milenic, L. H. Bryant, C. A. S. Regino and M. W. Brechbiel, J. Inorg. Biochem., 2011, 105, 722–727 CrossRef CAS.
- V. C. Pierre, M. Botta and K. N. Raymond, J. Am. Chem. Soc., 2005, 127, 504–505 CrossRef CAS.
- W. C. Floyd, III, P. J. Klemm, D. E. Smiles, A. C. Kohlgruber, V. C. Pierre, J. L. Mynar, J. M. J. Fréchet and K. N. Raymond, J. Am. Chem. Soc., 2011, 133, 2390–2393 CrossRef.
- G. Gugliotta, M. Botta and L. Tei, Org. Biomol. Chem., 2010, 8, 4569–4574 CAS.
- M. M. Ali, M. Woods, P. Caravan, A. C. L. Opina, M. Spiller, J. C. Fettinger and A. D. Sherry, Chem.–Eur. J., 2008, 14, 7250–7258 CrossRef CAS.
- D. A. Tomalia, L. A. Reyna and S. Svenson, Biochem. Soc. Trans., 2007, 35, 61–67 CrossRef CAS.
- Z. Cheng, D. L. J. Thorek and A. Tsourkas, Angew. Chem., Int. Ed., 2010, 49, 346–350 CrossRef CAS.
- W. Pham, B.-Q. Zhao, E. H. Lo, Z. Medarova, B. Rosen and A. Moore, NeuroImage, 2005, 28, 287–292 CrossRef.
- R. Gabathuler, Neurobiol. Dis., 2010, 37, 48–57 CrossRef CAS.
- C. A. Mathis, Y. Wang and W. E. Klunk, Curr. Pharm. Des., 2004, 10, 1469–1492 CrossRef CAS.
- L. Nichols, V. W. Pike, L. Cai and R. B. Innis, Biol. Psychiatry, 2006, 59, 940–947 CrossRef CAS.
- W. E. Klunk, H. Engler, A. Nordberg, Y. Wang, G. Blomqvist, D. P. Holt, M. Berström, I. Savitcheva, G. F. Huang, S. Estrada, B. Ausén, M. L. Debnath, J. Barletta, J. C. Price, J. Sandell, B. J. Lopresti, A. Wall, P. Koivisto, G. Antoni, C. A. Mathis and B. Långström, Ann. Neurol., 2004, 55, 306–319 CrossRef CAS.
- W. E. Klunk, J. C. Price, B. J. Lopresti, M. L. Debnath, D. P. Holt, Y. Wang, G. F. Huang, L. Shao, C. Meltzer, S. T. DeKosky and C. A. Mathis, Neurobiol. Aging, 2004, 25, S58 CrossRef.
- G. W. Small, V. Kepe, L. M. Ercoli, P. Siddarth, S. Y. Bookheimer, K. J. Miller, H. Lavretsky, A. C. Burggen, G. M. Cole, H. V. Vinters, P. M. Thompson, S.-C. Huang, N. Satyamurthy, M. E. Phelps and J. R. Barrio, N. Engl. J. Med., 2006, 355, 2652–2663 CrossRef CAS.
- N. P. Verhoeff, A. A. Wilson, S. Takeshita, L. Trop, D. Hussey, K. Singh, H. F. Kung, M. P. Kung and S. Houle, Am. J. Geriatr. Psychiatry, 2004, 12, 584–595 Search PubMed.
- L. Vander Elst, I. Raynal, M. Port, P. Tisnès and R. N. Muller, Eur. J. Inorg. Chem., 2005, 1142–1148 CrossRef CAS.
- P. Fries, V. M. Runge, A. Bücker, H. Schürholz, W. Reith, P. Robert, C. Jackon, T. Lanz and G. Schneider, Invest. Radiol., 2009, 44, 200–206 CrossRef.
- M. Beaumont, B. Lemasson, R. Farion, C. Segebarth, C. Rémy and E. L. Barbier, J. Cereb. Blood Flow Metab., 2009, 29, 1714–1726 CrossRef.
- Y. Z. Wadghiri, E. M. Sigurdsson, M. Sadowski, J. I. Elliott, Y. Li, H. Scholtzova, C. Y. Tang, G. Aguinaldo, M. Pappolla, K. Duff, T. Wisniewski and D. H. Turnbull, Magn. Reson. Med., 2003, 50, 293–302 CrossRef CAS.
- R. M. Koffie, C. T. Farrar, L.-J. Saidi, C. M. William, B. T. Hyman and T. L. Spires-Jones, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 18837–18842 CrossRef CAS.
- M. Demeule, J.-C. Currie, Y. Bertrand, C. Ché, T. Nguyen, A. Régina, R. Gabathuler, J. P. Castaigne and R. Béliveau, J. Neurochem., 2008, 106, 1534–1544 CrossRef CAS.
- B. V. Zlokovic, R. Deane, A. P. Sagare, R. D. Bell and E. A. Winkler, J. Neurochem., 2010, 115, 1077–1089 CrossRef CAS.
- J. F. Poduslo, G. L. Curran, J. A. Peterson, D. J. McCormick, A. H. Fauq, M. A. Khan and T. M. Wengenack, Biochemistry, 2004, 43, 6064–6075 CrossRef CAS.
- K. K. Kandimalla, T. M. Wengenack, G. L. Curran, E. J. Gilles and J. F. Poduslo, J. Pharmacol. Exp. Ther., 2007, 322, 541–549 CrossRef CAS.
- J. F. Poduslo, M. Ramakrishnan, S. S. Holasek, M. Ramirez-Alvarado, K. K. Kandimalla, E. J. Gilles, G. L. Curran and T. M. Wengenack, J. Neurochem., 2007, 102, 420–433 CrossRef CAS.
- M. Ramakrishnan, T. M. Wengenack, K. K. Kandimalla, G. L. Curran, E. J. Gilles, M. Ramirez-Alvarado, J. Lin, M. Garwood, C. R. Jack, Jr and J. F. Poduslo, Pharm. Res., 2008, 25, 1861–1872 CrossRef CAS.
- J. J. Min and S. S. Gambhir, Gene Ther., 2004, 11, 115–125 CAS.
| This journal is © The Royal Society of Chemistry 2012 |
