Reply to the ‘Comment on “Theoretical investigations on hydrogen peroxide decomposition in aquo”’ by W. H. Koppenol, Phys. Chem. Chem. Phys., 2021, 23, DOI: 10.1039/D1CP03545B
Abstract
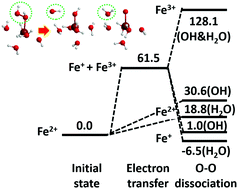
Following the comment article by Koppenol (W. H. Koppenol, Phys. Chem. Chem. Phys., 2021, 23, DOI: 10.1039/D1CP03545B), the H2O2 decomposition reaction mechanism based on the production of a de facto monovalent iron ion hydration complex, which is theoretically proposed in T. Tsuneda and T. Taketsugu, Phys. Chem. Chem. Phys., 2018, 20, 24992–24999, is reviewed from the standpoint of chemical kinetics. As a result, it is found that this decomposition mechanism is strongly supported by comparing the Gibbs energies of formation in consideration of the O–O bond dissociation of H2O2 for de facto monovalent iron, ferrous, and ferric ion complexes.


 Please wait while we load your content...
Please wait while we load your content...