Ni(ii)-Catalyzed enantioselective Mukaiyama–Mannich reaction between silyl enol ethers and cyclic N-sulfonyl α-ketiminoesters†
Abstract
The enantioselective Mukaiyama–Mannich reaction of cyclic N-sulfonyl α-ketiminoesters and silyl enol ethers was realized for the first time using a Ni(II)-bis(oxazoline) complex. Both di- and tri-substituted silyl enol ethers as well as silyl dienol ethers generated from the corresponding aromatic ketones or α,β-unsaturated ketones were well tolerated in this reaction. The reaction proceeded smoothly under mild conditions to provide a series of enantioenriched benzofused sultams containing an α-quaternary α-amino ester and an aromatic ketone moiety. Moreover, the products can be readily converted into potentially bioactive benzosultams including aziridine and polycyclic benzosultams.
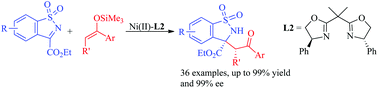

 Please wait while we load your content...
Please wait while we load your content...