Formic acid catalyzed isomerization of protonated cytosine: a lower barrier reaction for tautomer production of potential biological importance†
Abstract
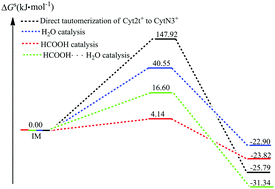
Tautomerism in nucleotide bases is one of the possible mechanisms of DNA mutation. In spite of numerous studies on the structure and energy of protonated cytosine tautomers, little information is available on the process of their intra- and intermolecular tautomerizations. The catalytic ability of H2O, HCOOH, and the HCOOH⋯H2O group to facilitate the tautomerism of the Cyt2t+ to CytN3+ isomer has been studied. It is shown that the activation free energies of tautomerism in the gas phase are 161.17, 58.96, 26.06, and 15.69 kJ mol−1, respectively, when the reaction is carried out in the absence and presence of H2O, HCOOH, or the HCOOH⋯H2O group. The formation of a doubly hydrogen bonded transition state is central to lowering the activation free energy and facilitating the intramolecular hydrogen atom transfer that is required for isomerization. In the aqueous phase, although the solvent effects of water significantly decrease the activation free energy of intramolecular tautomerization, the isomerization of the Cyt2t+ to CytN3+ isomer remains unfavorable, and the HCOOH and HCOOH⋯H2O group mediated mechanisms are still more favorable. Meanwhile, conventional transition state theory (CTST) followed by Wigner tunneling correction is then applied to estimate the rate constants. The rate constant with Wigner tunneling correction for direct tautomerization is obviously smaller than that of HCOOH-mediated tautomerization, which is the most plausible mechanism. Finally, another important finding is that the product complex (CytN3+⋯HCOOH) is in the rapid tautomeric equilibrium with the reaction complex (Cyt2t+⋯HCOOH) (τ99.9% = 3.84 × 10−12 s), which is implemented by the mechanism of the concerted synchronous double proton transfer. Its lifetime of the formed CytN3+⋯HCOOH complex (τ = 8.33 × 10−9 s) is almost one order of magnitude larger than the time required for the replication machinery to forcibly dissociate a base pair into the monomers during DNA replication (several ns), which is further dissociated into the CytN3+ and HCOOH monomers. The results of the present study demonstrate the feasibility of acid catalysis for DNA base isomerization reactions that would otherwise be forbidden.
- This article is part of the themed collection: 2017 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...