Probing keto–enol tautomerism using photoelectron spectroscopy†
Abstract
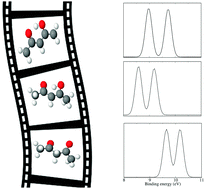
We theoretically investigate the mechanism of tautomerism in the gas-phase acetylacetone molecule. The minimum energy path between the enolone and diketo forms has been computed using the Nudged-Elastic Band (NEB) method within the density-functional theory (DFT) using the projector augmented-wave method and generalized gradient approximation in Perdew–Wang (PW91) parametrization. The lowest transition state as well as several intermediate geometries between the two stable tautomers have been identified. The outer-valence ionization spectra for all determined geometries have been computed using the third-order non-Dyson algebraic diagrammatic construction technique. Furthermore, the oxygen core–shell ionization spectra for these geometries have been obtained using DFT and the Becke three-parameter Lee–Yang–Parr (B3LYP) functional. It is shown that all spectra depend strongly on the geometries demonstrating the possibility of following the proton-transfer dynamics using photoelectron spectroscopy in pump–probe experiments.

 Please wait while we load your content...
Please wait while we load your content...