Highly selective, reversible water activation by P,N-cooperativity in pyridyl-functionalized phosphinines†‡
Abstract
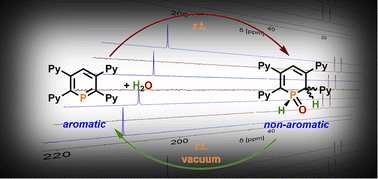
Tetrapyridyl-functionalized phosphinines were prepared and structurally characterized. The donor-functionalized aromatic phosphorus heterocycles react highly selectively and even reversibly with water. Calculations reveal P,N-cooperativity for this process, with the flanking pyridyl groups serving to kinetically enhance the formal oxidative addition process of H2O to the low-coordinate phosphorus atom via H-bonding. Subsequent tautomerization forms 1,2-dihydrophosphinine derivatives, which can be quantitatively converted back to the phosphinine by applying vacuum, even at room temperature. This process can be repeated numerous times, without any sign of decomposition of the phosphinine. In the presence of CuI·SMe2, dimeric species of the type ([Cu2I2(phosphinine)]2) are formed, in which each phosphorus atom shows the less common μ2-bridging 2e−-lone-pair-donation to two Cu(I)-centres. Our results demonstrate that fully unsaturated phosphorus heterocycles, containing reactive P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bonds, are interesting candidates for the activation of E–H bonds, while the aromaticity of such compounds plays an appreciable role in the reversibility of the reaction, supported by NICS calculations.
C double bonds, are interesting candidates for the activation of E–H bonds, while the aromaticity of such compounds plays an appreciable role in the reversibility of the reaction, supported by NICS calculations.
- This article is part of the themed collection: 2024 Chemical Science Covers


 Please wait while we load your content...
Please wait while we load your content...