Corannulene and its complex with water: a tiny cup of water†
Abstract
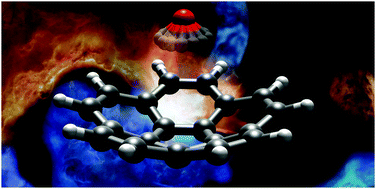
We report the results of a broadband rotational spectroscopic study of corannulene, C20H10, all of its singly substituted 13C isotopologues, and a complex of corannulene with one molecule of water. Corannulene is a polycyclic aromatic hydrocarbon (PAH) with a curved structure that results in a large dipole moment. Observation of 13C isotopic species in natural abundance allowed us to precisely determine the molecular structure of corannulene. The differences between the experimental C–C bond lengths correlate to the double-bond character predicted using Kekule's resonance structures. In the case of C20H10–H2O, the water molecule is found to reside inside the bowl-like structure of corannulene. Our experimental and theoretical results indicate that the water molecule rotates freely around its C2 axis and that dispersion interactions are the dominant contribution to the binding.


 Please wait while we load your content...
Please wait while we load your content...