Selective modification of the 3′′-amino group of kanamycin prevents significant loss of activity in resistant bacterial strains†
Abstract
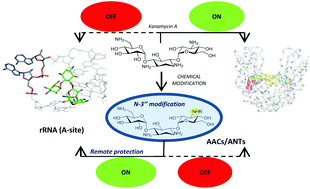
Aminoglycosides are highly potent, wide-spectrum bactericidals. N-1 modification of aminoglycosides has thus far been the best approach to regain bactericidal efficiency of this class of antibiotics against resistant bacterial strains. In the present study we have evaluated the effect that both, the number of modifications and their distribution on the aminoglycoside amino groups (N-1, N-3, N-6′ and N-3′′), have on the antibiotic activity. The modification of N-3′′ in the antibiotic kanamycin A is the key towards the design of new aminoglycoside antibiotics. This derivative maintains the antibiotic activity against aminoglycoside acetyl-transferase- and nucleotidyl-transferase-expressing strains, which are two of the most prevalent modifying enzymes found in aminoglycoside resistant bacteria.

 Please wait while we load your content...
Please wait while we load your content...