Dependence of the SBU length on the size of metal ions in alkaline earth MOFs derived from a flexible C3-symmetric tricarboxylic acid†
Abstract
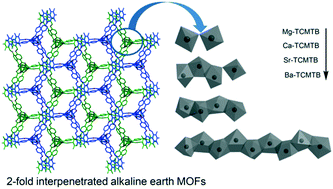
Four new alkaline-earth metal-based metal–organic frameworks, [Mg4(TCMTB)2(OAc)2(DMA)2(H2O)3]n (Mg-TCMTB), {[Ca4(TCMTB)2(OH)(DMF)2(H2O)5]·Cl}n (Ca-TCMTB), [Sr4(TCMTB)2(OH)(OAc)(DMA)6(H2O)]n (Sr-TCMTB) and [Ba9(TCMTB)4(NO3)6(DMA)14]n (Ba-TCMTB) (H3TCMTB = 2,4,6-tris[(4′-carboxyphenoxy)methyl]-1,3,5-trimethylbenzene), have been synthesized and structurally characterized. Structural analysis of the MOFs reveals the presence of diverse structures and topologies in these systems due to the conformational flexibility and multiple coordination sites in H3TCMTB. Coordination polymers Mg-TCMTB, Ca-TCMTB and Ba-TCMTB MOFs are three-dimensional frameworks exhibiting 2-fold interpenetration and one-dimensional hexagonal channels, while Sr-TCMTB is a 2-fold interpenetrated layered MOF. The 2D layers in Sr-TCMTB are interconnected through H–O⋯H hydrogen bonds. Increasing ionic radii and coordination number on moving down the group results in the formation of bi-, tetra- and nona-nuclear M–O–M connected inorganic building units. Owing to its smaller size and lower coordination number, framework Mg-TCMTB gives rise to a moderate surface area of 33.0 m2 g−1 (SABET) and 93.8 m2 g−1 (SALang) which is the highest observed among all the four MOFs. Emission studies of the new MOFs reveal the presence of strong photoluminescence at 380 nm.

 Please wait while we load your content...
Please wait while we load your content...