Saccharothriolides A–C, novel phenyl-substituted 10-membered macrolides isolated from a rare actinomycete Saccharothrix sp.†
Abstract
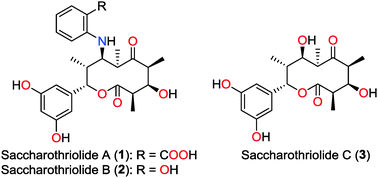
Three new 10-membered macrolides, saccharothriolides A–C (1–3), were discovered from a rare actinomycete Saccharothrix sp. A1506. All of the sp3 carbons in the 10-membered ring had chirality, which was determined by extensive spectroscopic analysis and TDDFT-calculation of ECD spectra. Saccharothriolide B (2) exhibited cytotoxicity against human tumor cell lines HeLa and HT1080.

 Please wait while we load your content...
Please wait while we load your content...