Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Anion transport and supramolecular medicinal chemistry
Philip A.
Gale
 *a,
Jeffery T.
Davis
*a,
Jeffery T.
Davis
 *b and
Roberto
Quesada
*b and
Roberto
Quesada
 *c
*c
aSchool of Chemistry (F11), The University of Sydney, 2006 NSW, Australia. E-mail: philip.gale@sydney.edu.au
bDepartment of Chemistry and Biochemistry, University of Maryland, College Park, MD 20742, USA. E-mail: jdavis@umd.edu
cDepartmento de Química, Universidad de Burgos, 09001 Burgos, Spain. E-mail: rquesada@ubu.es
First published on 5th April 2017
Abstract
New approaches to the transmembrane transport of anions are discussed in this review. Advances in the design of small molecule anion carriers are reviewed in addition to advances in the design of synthetic anion channels. The application of anion transporters to the potential future treatment of disease is discussed in the context of recent findings on the selectivity of anion transporters.
Introduction
Ion transport across biological membranes is an important process, regulated by membrane-embedded pumps, channels and carriers, that maintains ion and pH homeostasis in cells.1 Genetic diseases can result in dysregulated ion transport due to perturbations in the structure of ion channels.2 These diseases, such as cystic fibrosis (CF) caused by the dysfunction of the cystic fibrosis transmembrane conductance regulator (CFTR),3 are collectively known as channelopathies.4The development of synthetic carriers and transmembrane channels has been driven to some degree by the potential application of these supramolecular constructs in the treatment of diseases caused by dysregulated anion transport. Many groups have used model membrane systems often employing vesicles composed of lipid or lipid/cholesterol mixtures as test beds for the efficacy of synthetic transporters. Excitingly, more recently, groups have begun to explore the anion transport properties of synthetic ionophores in cells. Supramolecular medicinal chemistry5 – the development of supramolecular systems to function in biological systems with therapeutic benefit – is still at an early stage. Encouragingly anion transporters have been shown to be capable of transporting chloride through epithelial cell membranes – effectively replacing the function of faulty CFTR channels. pH gradient dissipation is often observed within cells upon exposure to anion transporters and recent work has elucidated the mechanism of this process which often leads to apoptosis. Hence anion transporters also have potential application in the treatment of cancer and natural products, such as the prodigiosins and synthetic analogues capable of H+/Cl− transport, have been studied extensively in this regard.
This tutorial review will cover recent progress in anion transporter design including new hydrogen bonding anionophores and systems that use non-classical interactions such as halogen bonding to complex and transport anionic substrates. We will also look at recent developments in the design and synthesis of synthetic channels and pores and also progress in optimising transport mediated by small molecules. New assays for monitoring anion transport and measuring selectivity in transport will also be discussed.
Synthetic hydrogen bond donors
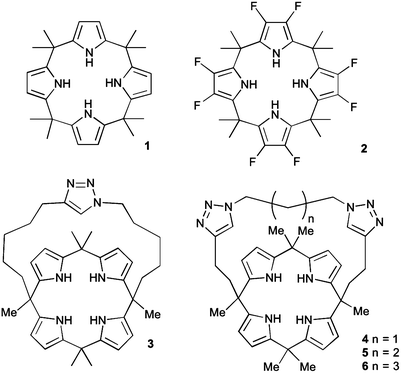
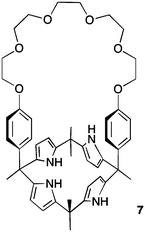
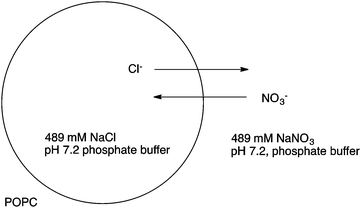
Calix[4]pyrrole macrocycles have demonstrated great versatility as platforms to develop transmembrane ion transporters. These macrocycles are capable of binding both anions and large, polarizable cations through the pyrrolic N–H groups and the aromatic cup respectively. Early studies showed the ability of meso-octamethylcalix[4]pyrrole 1 to promote CsCl transmembrane co-transport (symport). Co-transport or symport is the process in which two ions are transported together across a lipid bilayer. In the case of compound 1 the macrocycle functions as an electroneutral CsCl co-transporter as the overall transport process does not result in a net flow of charge across the membrane. Gale, Sessler et al. studied the parent meso-octamethyloctafluorocalix[4]pyrrole 2.6 Fluorination of the pyrrole rings was found to enhance the acidity of the NH hydrogen bond donor groups leading to stronger NH⋯Cl− interactions and dramatically change the transmembrane transport activity of the molecule. Thus compound 2 proved to be an efficient anion exchanger, promoting nitrate/chloride exchange in 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC) vesicles irrespective of the MCl (M = Li, Na, K, Rb, Cs) salt employed. This compound also facilitated bicarbonate/chloride exchange. Therefore, the presence of the electron-withdrawing fluorine substituents induces a change from CsCl co-transporter as predominant transport mechanism for 1 to a much more potent anion exchanger capable of anion uniport for 2. Uniport is the simple transport of one species across a membrane – in the case of compound 2 it is capable of chloride uniport in the absence of caesium cations. Strapped calix[4]pyrroles 3–6, bearing triazole groups that contribute to enhance the chloride binding affinity were studied by Gale.7 Chloride efflux from POPC liposomes was measured using several chloride salts. In all cases, higher chloride efflux was observed in the chloride/nitrate exchange assay using caesium salts. This result highlighted the contribution of both MCl co-transport and anion exchange to the transport activity displayed by these compounds. The length of the alkyl chain of the bis-triazole strap was found to be very important in the extent of chloride efflux, with compound 6 being the most effective (Fig. 1).The binding and transport properties of an oligoether-strapped calix[4]pyrrole 7 were studied by Gale, Sessler and Lee (Fig. 2).8 This compound formed stable 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ion-pair complexes with LiCl and NaCl. Chloride selective electrode assays in POPC liposomes showed that 7 can function both as a chloride/nitrate exchanger as well as M+/Cl− co-transporter, preferentially for caesium cations.
1 ion-pair complexes with LiCl and NaCl. Chloride selective electrode assays in POPC liposomes showed that 7 can function both as a chloride/nitrate exchanger as well as M+/Cl− co-transporter, preferentially for caesium cations.
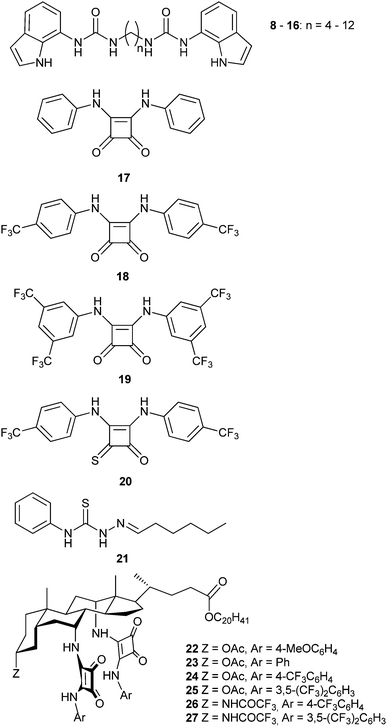
Simple bis-indolylurea derivatives 8–16 were studied by Gale and colleagues.9 The two anion binding units were linked through flexible alkyl chains of different length. Despite the lack of cooperativity of the binding sites, these compounds were found to function as effective anion transporters, outperforming the parent mono-indolylurea derivatives. The relative transport activity could be modulated by choosing the appropriate alkyl linker, which has a direct impact on the overall lipophilicity of the transporter. Molecular dynamics simulations in the POPC bilayer model indicated that the structure of the lipid bilayer is not disturbed by the internalization of these compounds and the average distance that a molecule travels inside the membrane was reduced for the longer transporter, which is in agreement with the lower activity of this compound.
The same group introduced squaramide-based compounds such as derivatives 17–19 as transmembrane anion transporters.10 These compounds outperformed analogous ureas and thioureas in terms of transmembrane transport activity. Using POPC liposomes, these compounds proved extremely efficient transporters promoting both nitrate/chloride exchange and bicarbonate/chloride exchange, with EC50 values (the effective concentration of transporter to achieve 50% anion efflux at a particular time in the experiment) down to 0.01% transporter![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
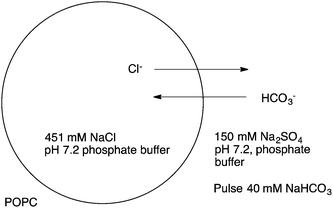
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) lipid molar ratio in the case of nitrate/chloride exchange facilitated by 19. The analogous oxothiosquaramide 20 was also developed and studied by Gale, Jolliffe and colleagues.11 The transmembrane anion transport abilities of this oxothiosquaramide were investigated in liposomes and it was found that it functions as mobile carrier that can promote chloride efflux mainly via chloride/nitrate exchange process, although HCl symport can also occur in the presence of a pH gradient. This compound is significantly more acidic than regular squaramides, with pKa values around 6. Thus at pH 7.2 the anion transport ability of 20 is fully switched OFF due to deprotonation of the receptor, but the activity is switched ON at lower pH. Gale's group have also provided another remarkable example of a pH switchable transporter.12 Phenylthiosemicarbazones can be protonated at acidic pH and function as a highly active H+/Cl− cotransporters. For instance, the calculated EC50 for compound 21 in the chloride/nitrate assay at pH 4.0 was 0.0074 mol%. This value is 640-times lower than that observed at pH of 7.2. Thus, this system represents an example of a non-electrogenic (or electroneutral) transporter (a transporter that does not facilitate a net flow of charge as – in this case – both a H+ and Cl− are transported) displaying pH-switching behaviour between acidic and neutral pH conditions. This pH switchable activity has potential useful implications in biological studies, targeting for instance chloride transport in acidic organelles (Fig. 3).
lipid molar ratio in the case of nitrate/chloride exchange facilitated by 19. The analogous oxothiosquaramide 20 was also developed and studied by Gale, Jolliffe and colleagues.11 The transmembrane anion transport abilities of this oxothiosquaramide were investigated in liposomes and it was found that it functions as mobile carrier that can promote chloride efflux mainly via chloride/nitrate exchange process, although HCl symport can also occur in the presence of a pH gradient. This compound is significantly more acidic than regular squaramides, with pKa values around 6. Thus at pH 7.2 the anion transport ability of 20 is fully switched OFF due to deprotonation of the receptor, but the activity is switched ON at lower pH. Gale's group have also provided another remarkable example of a pH switchable transporter.12 Phenylthiosemicarbazones can be protonated at acidic pH and function as a highly active H+/Cl− cotransporters. For instance, the calculated EC50 for compound 21 in the chloride/nitrate assay at pH 4.0 was 0.0074 mol%. This value is 640-times lower than that observed at pH of 7.2. Thus, this system represents an example of a non-electrogenic (or electroneutral) transporter (a transporter that does not facilitate a net flow of charge as – in this case – both a H+ and Cl− are transported) displaying pH-switching behaviour between acidic and neutral pH conditions. This pH switchable activity has potential useful implications in biological studies, targeting for instance chloride transport in acidic organelles (Fig. 3).
Squaramide binding motifs were also appended to the axial positions of steroid-based anion receptors (compounds 22–27, Fig. 3) by Gale and A. P. Davis.13 Steroid structures (in particular cholic acid derivatives) are lipophilic motifs developed by A. P. Davis's group as lipophilic scaffolds from which anion transporters may be constructed. In this case, the steroid framework with squaramide binding units resulted in extremely high affinities for tetra-alkylammonium salts of anions in chloroform. These compounds were found to be active transporters in POPC liposomes at loadings of receptor/lipid = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2500 by the lucigenin method (lucigenin's fluorescence is collisionally quenched by halide anions allowing transmembrane transport to be followed by fluorescence measurements).14 This activity is significantly lower than that found in thiourea decorated steroid-based anion receptors. This result could suggest that the affinity of these squaramide derivatives was too high and decomplexation of the anions is likely limiting the transmembrane transport activity of these compounds.
2500 by the lucigenin method (lucigenin's fluorescence is collisionally quenched by halide anions allowing transmembrane transport to be followed by fluorescence measurements).14 This activity is significantly lower than that found in thiourea decorated steroid-based anion receptors. This result could suggest that the affinity of these squaramide derivatives was too high and decomplexation of the anions is likely limiting the transmembrane transport activity of these compounds.
Channels and pores
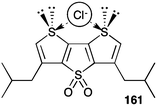
Protein channels that span the bilayer membrane provide a conduit for charged ions to cross that hydrophobic barrier. Defects in these channel proteins can lead to serious diseases, such as cystic fibrosis. One strategy for combating such “channelopathies” would be to use a synthetic compound that could arrange in the bilayer membrane so as to mimic the structure and function of an anion channel. In the past 5–6 years there have been a number of synthetic channels designed for the specific purpose of catalysing the transmembrane transport of anions. Most of these synthetic anion channels have been formed by self-assembly of molecular building blocks within the lipid membrane. The ultimate proof of an ion channel is usually taken to be the “open–closed” ion conductances shown in voltage clamp experiments on planar bilayers. The interpretation is that this conductance is due to the dynamic self-assembly of subunits within the membrane. When an active structure is formed by self-assembly then a transient pathway is formed that enables anion conductance across the membrane.Gokel and colleagues described a series of low molecular weight isophthalamides and pyridine analogues that functioned as transmembrane Cl− transporters in POPC vesicles.15 While most of the compounds described in the paper were mobile carriers and not channel formers, 4,4′-dinitropicolinanilide 28 (m.w. 407) showed properties characteristic of a functional ion channel (Fig. 4) at voltages that are physiologically relevant. The most convincing evidence for channel formation by 28 was its hallmark “open–closed” conductance activity in planar bilayer experiments. The authors found that the most frequently observed conductance state corresponded to a pore size of ca. 8 Å, close to the diameter of a hydrated Cl− anion (6.5 Å). To function as a channel, the small molecule 28 would have to self-assemble into an ordered pore within the hydrophobic membrane. Gokel and colleagues suggested that 28 self-assembles via π–π interactions between individual subunits. In support of such a mechanism the authors offered 3 pieces of evidence. First, an X-ray crystal structure of 28 shows that it forms π–π stacks in the solid-state. Second, fluorescence spectra of 28 in the presence of 0.17 mM DOPC vesicles showed formation of an excimer peak that the authors proposed formed upon self-association in the lipid membrane. Finally, using data from anion transport studies in POPC vesicles, the authors found that 4,4′-dinitropicolinanilide 28 transported Cl− with a Hill coefficient of >1 at relatively high concentrations of added transporter. Such a value for the Hill coefficient would indicate that multiple monomers come together to form the active transporter. Gokel and colleagues built an energy-minimized model of the channel by stacking triarenes in a face-to-face orientation; they proposed that this “semi-organized” array of stacked hydrogen bond sites could catalyse transmembrane transport of Cl− anions. They suggested that sliding or rotation of an individual monomer 28 out of the stacked array could account for the “open–closed” conductance observed in the bilayer conductance experiments. Even though the proposed channel structure is speculative this study nicely highlights how self-assembly of low molecular weight building blocks can be a powerful strategy for construction of synthetic anion channels that function in lipid membranes.
 | ||
| Fig. 4 Transmembrane chloride transport by (a) 4,4′-dinitropicolinamide 28 appears to occur via a channel mechanism: (b) the current observed at applied voltages of −60 mV and +60 mV upon addition of transporter 28 to a planar bilayer (c) DFT-calculated molecular model showing a stack of 10 molecules of 28, 3 chloride anions and 16 water molecules. The distance between the most distal monomers in the stack is approximately 34 Å. Reproduced from ref. 15. Copyright 2010, Royal Society of Chemistry. | ||
Recently, Gong and colleagues have extended this self-assembly strategy to form transmembrane ion channels from aromatic oligoamide macrocycles, such as 29-H and 29-NH2.16 The authors hypothesized that a transmembrane pore would form if about 10 macrocycles stacked one on another to give a nanotube of ca. 34 Å, a distance matching the thickness of the hydrophobic portion of the bilayer membrane. Furthermore such a nanotube, formed by self-assembly of macrocycles 29, would also contain ca. 10 functional groups on its inner surface. The authors reasoned that the identity of these functional groups (shown as X in Fig. 5) on the channel's interior might well influence their ion selectivity.
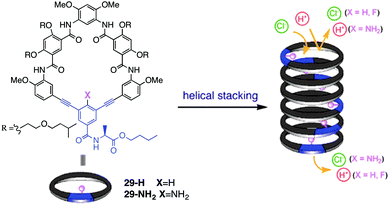 | ||
| Fig. 5 Structure of oligoamide macrocycles 29-H and 29-NH2 and self-assembly of monomers into nanotubular pores that conduct ions across lipid membranes. The identity of the –X group on the interior of the pore influences the ion transport selectivity of the self-assembled ion channel. Reproduced from ref. 16. Copyright 2016, American Chemical Society. | ||
Both 29-H and 29-NH2 analogues were shown to self-assemble in non-polar solvents. Fluorescence spectra of 29-H in CHCl3 showed a broad band at 455 nm attributed to “excimer-like” emission caused by face-to-face stacking of aromatic rings in the resulting nanotubular structures. Atomic force microscopy also provided images consistent with formation of tubular stacks, with pore diameters of 2.8 ± 0.2 nm for 29-H and 2.7 ± 0.2 nm for 29-NH2.
Importantly, the authors found that macrocycles 29-H and 29-NH2 have different propensities for transporting chloride across lipid bilayers. They monitored transmembrane chloride transport across vesicles membranes using a Cl−-sensitive dye, N-(ethoxycarbonylmethyl)-6-methoxyquinoliniumbromide. This assay revealed that 29-NH2 was a relatively effective Cl− transporter and the authors proposed that this selectivity was due to the numerous amino groups that would be found on the nanotube's interior, each with a significant partially positive charge that would interact favourably with chloride anions. The authors obtained firm evidence for formation of transmembrane channels by 29-H and 29-NH2 in a series of single-channel conductance experiments using planar lipid bilayers. Both 29-H and 29-NH2 showed long-lasting, single conductance states, with respective conductance values of 238 ± 60 and 231 ± 44 pS, values that are entirely consistent with the nm-sized pores.
Most importantly in the context of designing anion-selective channels, the K+/Cl− transport selectivity for 29-H and 29-NH2, calculated from the ionic flux across a membrane as a function of the transmembrane potential and the concentrations of the ions inside and outside of the vesicle, revealed permeability ratios (PK+/PCl−) of 9 for the 29-H channel and a reduced value of 3.5 for the 29-NH2 channel. Even though both channels were actually cation-selective, the 29-NH2 channel showed a significant enhancement in its ability to conduct chloride anions across a membrane.
This work by Gong et al. has clearly shown how self-assembly of nanotubes with the same outer surface but with different internal functional groups can be used to modulate transport selectively based on charge. Further understanding of the mechanisms that control ion selectively will undoubtedly lead to improved design of future generation transporters that show enhanced transport selectivity for Cl− and other anions.
An example of a rosette motif in the design of self-assembled anion channels comes from the recent work of Talukdar and colleagues (Fig. 6).17 These researchers reasoned that the hydroxyl group, which can hydrogen bond with anions, might be useful for designing anion selective channels. The crystal structure of the diketal mannitol 30 showed an infinite chain formed by hydrogen-bonded dimers of 302, indicating that this compound has a strong propensity to self-associate in a nonpolar environment. The authors further suggested that stacks of cyclic rosettes formed from 3 or 4 units, 303 and 304, might well provide a transmembrane channel where anions could hop from one layer to the next by forming multivalent O–H⋯A–hydrogen bonds between adjacent layers.
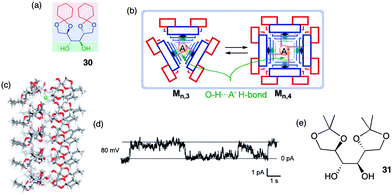 | ||
| Fig. 6 (a) Structure of mannitol transporter 30. (b) Schematic of how 30 self-assembles in lipid membrane. (c) Side-view of optimized structure of a 3 × 5 assembly of 30 containing a single Cl− anion. (d) Single-channel current traces recorded at 80 mV holding potential. (e) Inactive analogue 31. The main conductance state is indicated by dotted lines. Reproduced from ref. 17. Copyright 2014, American Chemical Society. | ||
Indeed, the protected mannitol 30 (m.w. = 342) promoted the transmembrane transport of anions. The authors first used the classical HPTS assay, which monitors the internal pH of a phospholipid liposome, to show that cyclohexylidene 30, but not an isopropylidene analogue 31 (Fig. 6e), readily dissipates a pH gradient across the DPPC vesicle. The authors attributed the anion transport activity by 30 to a fine balance between hydrophilic interactions, which enable hydrogen-bonding assembly of subunits into a pore, and hydrophobic interactions between the compound's cyclohexyl sidechains and the lipid bilayer, which allows for effective partitioning of 30 into the membrane. Analysis of transport kinetics as a function of transporter concentration revealed an impressive EC50 value of 42.5 μM and a Hill coefficient of k = 0.9. This Hill coefficient, which is close to 1, was interpreted to indicate that the active transporter was likely a multimeric assembly that self-associates with a high affinity.
A series of HPTS assays, done as a function of extravesicular anions, showed a halide selectivity of Cl− > Br− > I−, which the authors suggested meant that diol 30 preferred to bind Cl− by forming a series of intermolecular hydrogen bonds. Together the liposomal assays suggested that mannitol derivative 30 is a Cl− selective anion transporter. These data, however, cannot conclusively determine whether 30 functions as a unimolecular carrier (especially given the Hill coefficient of k = 0.9) or as a self-assembled channel, as might be suggested from the solid-state evidence for self-assembly of 30. Once again, single channel conductance experiments were used to demonstrate that 30 can function as a self-assembled channel. Distinct single-channel opening and closing events were observed and a single-channel conductance of 38.1 ± 3 pS was determined, which corresponds to a channel diameter of 3.06 Å. This estimate is close to the diameter of a dehydrated Cl− (d = 3.34 Å). To determine the channel diameter using the Hille equation the length of the channel formed must be known. In this work, this is estimated to be 34 Å as accurate measurements cannot be made. A small change in the channel's length could account for the 10% discrepancy between the two estimated values. Molecular models of a channel based on stacks of cyclic trimers showed a diameter of 3.23 Å, which agreed with the channel diameter of 3.06 Å obtained from the single-channel conductance measurements. Molecular dynamics calculations on a trimeric channel embedded in a slab of DPPC membrane also supported a hopping mechanism for Cl− transport; the anion makes and breaks O–H⋯Cl− hydrogen bonds as it moves through the channel via a relay mechanism, being passed along from one trimeric rosette to the next layer of –OH groups.
More recently, Talukdar's group has again demonstrated that a small molecule, this time the bis-diol 32 shown in Fig. 7, can be used for the self-assembly of a synthetic anion channel.18 Formation of the active structure is proposed to be due to intermolecular hydrogen bonds between the –OH hydroxyl groups on neighboring molecules. Anion transport activity, which was again determined using the HPTS base-pulse fluoresence assay, showed that the identity of the alkyl chains on the amine group of 32 was important for Cl−/OH− exchange, as efficient rates of anion transport required an optimal log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P values. Single-channel conductance experiments in planar bilayers clearly showed that 32 formed ion channels in DPhPC membranes, as depicted in Fig. 7c. Energy-minimized models of the self-assembled channel of 32n showed a nanotube lined with hydroxyl groups that point toward the nanotube's cavity and are well oriented for binding and transporting Cl− anion. In this paper, the authors also carried out extensive biological studies using bis-diol 32. They found that 32 could transport Cl− into cells and also trigger cell death in a number of different cell lines. Furthermore, a battery of experiments using various dye-based assays and confocal microscopy showed that Cl− transport catalyzed by bis-diol 32 disturbed the electrical potential across mitochondrial membranes, which subsequently led to generation of reactive oxygen species and triggered release of cytochrome c from the mitochondria. The authors concluded that this type of cell death caused by 32 was due to apoptosis, as was supported by the over-expression of caspase proteins upon treatment of cells with bis-diol 32. The ability of small molecule such as bis-diol 32 to induce apoptosis, presumably via a chloride transport process, may have therapeutic potential in treatments of various diseases.
P values. Single-channel conductance experiments in planar bilayers clearly showed that 32 formed ion channels in DPhPC membranes, as depicted in Fig. 7c. Energy-minimized models of the self-assembled channel of 32n showed a nanotube lined with hydroxyl groups that point toward the nanotube's cavity and are well oriented for binding and transporting Cl− anion. In this paper, the authors also carried out extensive biological studies using bis-diol 32. They found that 32 could transport Cl− into cells and also trigger cell death in a number of different cell lines. Furthermore, a battery of experiments using various dye-based assays and confocal microscopy showed that Cl− transport catalyzed by bis-diol 32 disturbed the electrical potential across mitochondrial membranes, which subsequently led to generation of reactive oxygen species and triggered release of cytochrome c from the mitochondria. The authors concluded that this type of cell death caused by 32 was due to apoptosis, as was supported by the over-expression of caspase proteins upon treatment of cells with bis-diol 32. The ability of small molecule such as bis-diol 32 to induce apoptosis, presumably via a chloride transport process, may have therapeutic potential in treatments of various diseases.
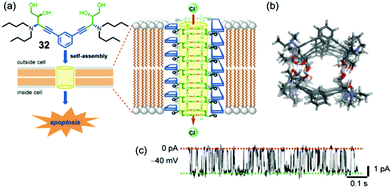 | ||
| Fig. 7 (a) Schematic depicting of a self-assembly of bis-diol 32 in a lipid membrane. The Cl− selective channel that spans the membrane is proposed to consist of stacks of hydrogen bonded dimers of 322. Transmembrane transport of chloride anions, which triggers apoptosis in a number of tested cell lines, is mediated by O–H⋯Cl− hydrogen bonds. (b) Top view of optimized structure of the anion channel formed by self-assembly of bis-diol 32. (c) Single-channel current traces recorded at −40 mV holding potential in 1 M symmetrical KCl solution. The main conductance state is indicated by dotted lines. Reproduced from ref. 18. Copyright 2016, American Chemical Society. | ||
Vargas Jentzch and Matile reported that rigid-rod scaffolds outfitted with tetrafluoroiodobenzyl sidechains catalyzed the transmembrane transport of Cl− and NO3− anions19 although they did not explicitly claim formation of transmembrane channels. Octamer 33, whose length approaches that of the hydrophobic bilayer, was the most active transporter in the series. The authors attributed the anion transport activity to the ability of these oligomers to provide a “hopping” pathway, whereby the anions can bind reversibly using both anion–π interactions and also halogen bonds with the σ-holes of the iodine atoms (Fig. 8). Matile's group had previously shown that halogen bonds in small molecules, such as pentafluoroiodobenzene or a calix[4]arene with four halogen-bond donors, could help transport anions, albeit relatively inefficiently, across membranes. The authors reasoned that anions likely bind to the pentafluoroiodobenzene monomer too weakly to allow for efficient transport; and anions bind too strongly to the convergent donors on the calix[4]arene macrocycle to allow high transport rates. To address this problem the authors used linear p-oligophenyl oligomers, compounds that can presumably self-assemble and span the hydrophobic membrane, so as to provide a kinetically feasible pathway for chloride anions to hop from one binding site to another as they move across the membrane.
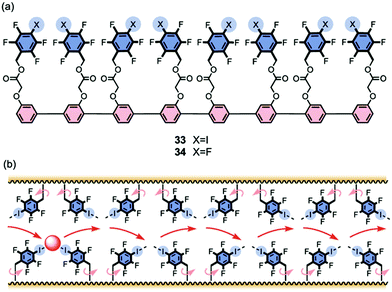 | ||
| Fig. 8 (a) Structures of rigid-rod transporters 33 and 34. Octamer 33, the most active transporter studied, is proposed to use both anion–π and halogen bond interactions to facilitate membrane transport of anions. (b) Schematic representation of how halogen bonding enables hopping of the anion from one binding site to the next as it moves through the lipid membrane. Reproduced from ref. 19. Copyright 2013, American Chemical Society. | ||
The authors used the classical HPTS assay to monitor Cl−/OH− exchange across large unilamellar vesicles (LUVs) made from egg yolk phosphatidylcholine (EYPC). Compounds such as 33 that contain the halogen-bonding motif, tetrafluoroiodo sidechains, were better anion transporters (by 3.5–26 fold) than those with pentafluorobenzyl groups, such as 34, which could only provide anion–π interactions. Importantly, the activity of both the anion–π and the halogen-bond transporters increased with length, reaching a maximum when the rods were the right length to span the hydrophobic section of the bilayer. Thus, the octamer transporter 33 showed an EC50 value of just 100 nM and had an impressive cooperativity coefficient of m = 3.37, making it 2360 times more active than monomeric pentafluoroiodobenzene. This study demonstrated that compounds that combine the attractive characteristics of the rigid-rod backbone with appendages providing transient anion binding sites using anion–π interactions and halogen bonds are quite impressive anion transporters. Presumably these compounds self-assemble to give bundles that help anions skip across the membrane.
One example of an unimolecular channel that conducts Cl− anions was recently described by Hou, Li and colleagues.20 They reported that relatively low concentrations (EC50 = 0.002 mol%) of pillar[n]arenes (n = 5, 6) with multiple peptide sidechains attached to the central core were able to transport amino acids across EYPC vesicles and do so enantioselectively for certain amino acids. They proposed that these compounds, which contained repeats of the non-polar phenylalanine in the peptide arms, folded into unimolecular channels within the bilayer membranes. A series of 1H NMR experiments in CD2Cl2 revealed that the peptide chains of 35 formed strong intramolecular hydrogen-bonds in this non-polar solvent. Similarly, infrared studies of these compounds embedded in EYPC vesicles also supported formation of an intramolecular hydrogen-bonded fold within the membrane. Energy-minimized models showed that these pillar[n]arenes formed a tubular structure, suggestive of a channel. While the paper's focus was on the unique ability of these pillar[n]arenes to transport amino acids across membranes, the authors noted that some natural amino acid transporters are also able to transport chloride anions across cell membranes. Indeed, by using the Cl− sensitive dye lucigenin Hou and colleagues demonstrated that compounds 35 and 36 also catalyzed transmembrane Cl− transport across EYPC membranes. Although more studies need to be done to confirm channel activity and determine anion selectivity, this interesting result demonstrates that the pillarene motif could be quite useful for building unimolecular anion channels (Fig. 9).
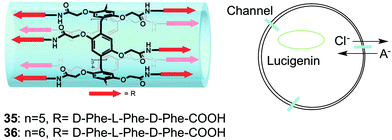 | ||
| Fig. 9 Schematic representations of pillararene transporters 35 and 36 (left) and representation of transmembrane Cl-transport mediated by compounds 35 and 36. Adapted from ref. 20. Copyright 2013, American Chemical Society. | ||
Optimising transport
The development of efficient anion transporters (i.e. compounds that have low EC50 values or high initial chloride efflux rates and hence can be used in low concentration) has attracted much interest and effort over recent years. By drawing an analogy to drug delivery and medicinal chemistry a number of research groups have made the link between the efficiency of a transporter and its lipophilicity. This is illustrated by Quesada and co-workers' study of the role of lipophilicity in anion transport processes mediated by tambjamines (Fig. 10).21 The chloride/bicarbonate antiport properties of tambjamines 37–52, that span a wide range of log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P values (n-octanol/water partition coefficient), were studied in POPC vesicles (Fig. 11) using a chloride selective electrode to monitor chloride flux from the vesicles. Separate experiments using 13C labelled HCO3− were used to confirm directly bicarbonate transport. The initial rate of chloride transport in the ion selective electrode experiments was plotted against calculated log
P values (n-octanol/water partition coefficient), were studied in POPC vesicles (Fig. 11) using a chloride selective electrode to monitor chloride flux from the vesicles. Separate experiments using 13C labelled HCO3− were used to confirm directly bicarbonate transport. The initial rate of chloride transport in the ion selective electrode experiments was plotted against calculated log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P (Fig. 12) showing a parabolic relationship between log
P (Fig. 12) showing a parabolic relationship between log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P and initial chloride transport rate and an optimum log
P and initial chloride transport rate and an optimum log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P for transport of around 4.2. The authors hypothesise that the compounds with lower log
P for transport of around 4.2. The authors hypothesise that the compounds with lower log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ps are too hydrophilic to partition effectively into the lipid bilayer than compounds with optimal log
Ps are too hydrophilic to partition effectively into the lipid bilayer than compounds with optimal log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P values. The lower activity of the compounds with higher log
P values. The lower activity of the compounds with higher log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P values was attributed to poorer deliverability of these compounds from the aqueous phase to the lipid bilayer and/or lower mobility of these tambjamines within the lipid bilayer. This relationship is very well known to medicinal chemists in which the activity of a drug has an optimal value and a parabolic relationship with log
P values was attributed to poorer deliverability of these compounds from the aqueous phase to the lipid bilayer and/or lower mobility of these tambjamines within the lipid bilayer. This relationship is very well known to medicinal chemists in which the activity of a drug has an optimal value and a parabolic relationship with log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P.
P.
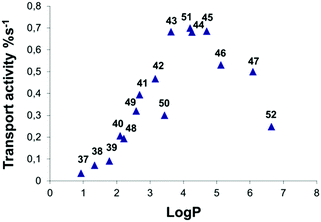 | ||
Fig. 12 Representation of transport activity, measured as initial chloride efflux (%s−1) vs. calculated average log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P for compounds 37–52. P for compounds 37–52. | ||
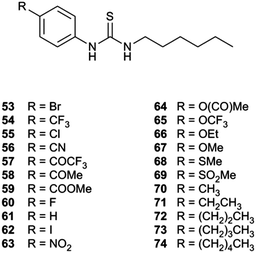
Gale and co-workers synthesized an extended series of 1-hexyl-3-phenylthioureas 53–74 with a variety of substituents on the 4-position of the phenyl ring (Fig. 13).22 These substituents were chosen to have a range of lipophilicities and electron withdrawing or electron donating effects. A Hill analysis was conducted with each of the transporters giving an EC50 for chloride/nitrate exchange (Fig. 14). A quantitative structure–activity relationship study was performed using Hansch analysis to determine the molecular parameters that are key in determining how a receptor will perform as an anion transporter.
The analysis resulted in eqn (1) and (2) being obtained. The first term in each equation relates to lipophilicity (retention time, RT or log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P) and has a positive effect of transport. Retention time on a reverse phase HPLC column is related to the lipophilicity of the compound. So in this set of compounds an increase in lipophilicity results in increased anion transport ability. The second term in both equations is the Hammett coefficient of the substituent on the 4-position of the phenyl ring. The positive sign of this term means that as the Hammett coefficient increases so the affinity of the thiourea for anionic guests increases as does its anion transport ability. The next term SPAN is related to the size of the molecule. This term is presumably related to diffusion of the transporter through the membrane with the negative sign meaning that as the molecule gets bigger its transport ability decreases. The relative importance of each term is shown graphically in Fig. 15 which highlights the dominance of lipophilicity in determining anion transport ability.
P) and has a positive effect of transport. Retention time on a reverse phase HPLC column is related to the lipophilicity of the compound. So in this set of compounds an increase in lipophilicity results in increased anion transport ability. The second term in both equations is the Hammett coefficient of the substituent on the 4-position of the phenyl ring. The positive sign of this term means that as the Hammett coefficient increases so the affinity of the thiourea for anionic guests increases as does its anion transport ability. The next term SPAN is related to the size of the molecule. This term is presumably related to diffusion of the transporter through the membrane with the negative sign meaning that as the molecule gets bigger its transport ability decreases. The relative importance of each term is shown graphically in Fig. 15 which highlights the dominance of lipophilicity in determining anion transport ability.
| log(1 /EC50) = 0.94(±0.07) × RT + 0.48(±0.14) × σp − 0.31(±0.07) × SPAN − 9.0(±0.8) N = 18, R2 = 0.93, Radj2 = 0.92, RMSE = 0.17, F = 68 | (1) |
log(1/EC50) = 0.81(±0.08) × log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P + 0.65(±0.19) × σp − 0.29(±0.09) × SPAN − 0.73(±0.79) N = 18, R2 = 0.89, Radj2 = 0.87, RMSE = 0.21, F = 39 P + 0.65(±0.19) × σp − 0.29(±0.09) × SPAN − 0.73(±0.79) N = 18, R2 = 0.89, Radj2 = 0.87, RMSE = 0.21, F = 39 | (2) |
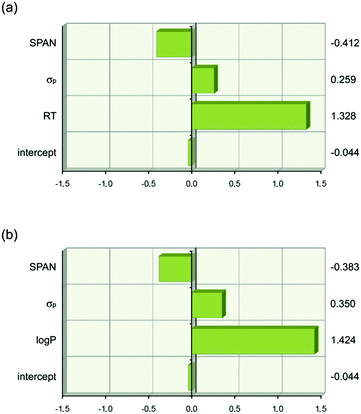 | ||
Fig. 15 Graphical depiction of the values of the coefficients in eqn (1) and (2) when the descriptor values are scaled to have a mean of zero and a range of two using JMP 9.0.0. This shows that lipophilicity (RT or log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P) has the strongest effect on anion transport. The values of the scaled coefficients for each descriptor are shown on the right hand side. (a) Eqn (1). (b) Eqn (2). P) has the strongest effect on anion transport. The values of the scaled coefficients for each descriptor are shown on the right hand side. (a) Eqn (1). (b) Eqn (2). | ||
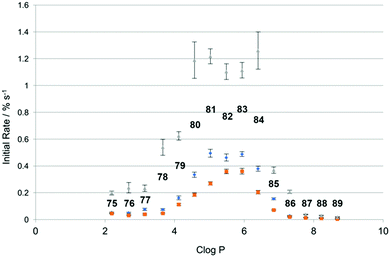
More recently, Spooner and Gale have investigated a set of similar thioureas 75–89 (Fig. 16) with a wider range of lipophilicities (Table 1) in lipid bilayers consisting of a number of different lipids (Fig. 17).23 The chloride/nitrate antiport properties of these compounds were measured under the conditions shown in Fig. 14 in a variety of different lipids and mixtures of lipids including POPC, POPG, and 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 POPE
1 POPE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) POPC. The initial rate of chloride transport was plotted against clog
POPC. The initial rate of chloride transport was plotted against clog![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P (Fig. 18) and it was found that under the three sets of conditions whilst the initial rate would vary the optimal log
P (Fig. 18) and it was found that under the three sets of conditions whilst the initial rate would vary the optimal log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P for transport remained constant.
P for transport remained constant.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P values for compounds 75–89
P values for compounds 75–89
| Cmpd | R1 | R2 | clog![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P P |
Cmpd | R1 | R2 | clog![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P P |
|---|---|---|---|---|---|---|---|
| 75 | H | Et | 2.19 | 83 | Pe | Pe | 5.94 |
| 76 | Me | Et | 2.68 | 84 | Pe | Hx | 6.40 |
| 77 | Et | Et | 3.14 | 85 | Hx | Hx | 6.85 |
| 78 | Et | Pr | 3.66 | 86 | Hx | Hp | 7.31 |
| 79 | Pr | Pr | 4.12 | 87 | Hp | Hp | 7.77 |
| 80 | Pr | Bu | 4.57 | 88 | Hp | Oc | 8.22 |
| 81 | Bu | Bu | 5.03 | 89 | Oc | Oc | 8.68 |
| 82 | Bu | Pe | 5.49 |
Following on from Gale's work on tren-based tripodal ureas and thioureas,24 this group decided to prepare very simple compounds containing a single urea or thiourea group and assess the ability of these systems to mediate chloride/nitrate and chloride/bicarbonate antiport (Fig. 19).25 Smaller molecules may obey ‘Lipinski's rule of 5’ and hence are more likely to have acceptable ADME (absorption, distribution, metabolism and excretion) properties. Transport experiments were run under the conditions shown in Fig. 14 for chloride/nitrate exchange and by the bicarbonate pulse method discussed above for chloride/bicarbonate. Carbon-13 NMR experiments with H13CO3− were used to confirm directly bicarbonate transport. The set of ureas proved ineffective at mediating both chloride/nitrate and chloride/bicarbonate exchange. However, the more lipophilic thioureas were effective at mediating both processes with the lowest EC50 values observed with compound 95 (Table 2). Compound 95, which combines a higher clog![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P and lower polar surface area than its inactive urea analogue, proved to be a remarkably potent anion transporter functioning in concentrations as low as 1
P and lower polar surface area than its inactive urea analogue, proved to be a remarkably potent anion transporter functioning in concentrations as low as 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25
25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 carrier to lipid ratio for chloride/nitrate exchange.
000 carrier to lipid ratio for chloride/nitrate exchange.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P and TPSA (Å2) are also presented for compounds 90–95
P and TPSA (Å2) are also presented for compounds 90–95
| Compounds | EC50 at 270 s (Cl−/NO3−) | Initial rate of chloride release (Cl−/NO3−)% Cl− efflux/s at 2% carrier loading | EC50 at 270 s (Cl−/HCO3−) | Initial rate of chloride release (Cl−/HCO3−)% Cl− efflux/s at 2% carrier loading | clog![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pa Pa |
PSAb (Å2) |
|---|---|---|---|---|---|---|
a clog![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P calculated using Spartan '08 for Macintosh (Ghose–Crippen model).
b Polar surface area (PSA) calculated using Spartan '08 for Macintosh. The receptors were minimised using AM1 semi-empirical methods with the two urea or thiourea NH groups parallel and the PSA and log P calculated using Spartan '08 for Macintosh (Ghose–Crippen model).
b Polar surface area (PSA) calculated using Spartan '08 for Macintosh. The receptors were minimised using AM1 semi-empirical methods with the two urea or thiourea NH groups parallel and the PSA and log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P values calculated. In the case of the case of the indole containing species two conformations were minimised – one with the indole NH forming a convergent array with the urea NH groups and the other with the indole NH oriented towards the urea or thiourea O or S atom (hence a range of values for PSA are given). P values calculated. In the case of the case of the indole containing species two conformations were minimised – one with the indole NH forming a convergent array with the urea NH groups and the other with the indole NH oriented towards the urea or thiourea O or S atom (hence a range of values for PSA are given).
|
||||||
| 90 | — | — | — | — | 1.99 | 37.0 |
| 91 | 0.1491% | 0.431 | 0.6049% | 0.227 | 3.14 | 22.2 |
| 92 | — | — | — | — | 2.42 | 34.8 |
| 93 | 3.0667% | 0.074 | 2.7848% | 0.188 | 3.57 | 21.5 |
| 94 | — | — | — | — | 2.02 | 44.6–47.7 |
| 95 | 0.02663% | 0.614 | 0.0405% | 0.386 | 3.16 | 31.3–35.5 |
Previous work by Gale had shown that fluorinated tren-based transporters were more effective than their parent analogues.26 Therefore series of fluorinated and non-fluorinated indole ureas and thioureas 96–99 were synthesised and their anion transport properties compared. The binding mode of compound 98 with chloride was revealed in the X-ray crystal structure of the tetrabutylammonium chloride salt of this receptor (Fig. 20). Compound 98 forms three N–H hydrogen bonds with the chloride ion (N–Cl distances 3.140(5)–3.406(5) Å, N–H⋯Cl angles 151.2–170.1°).
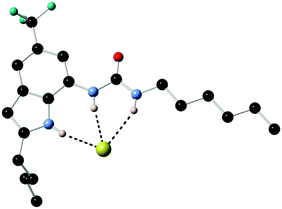 | ||
| Fig. 20 X-ray crystal structure of the chloride complex of 98. Non-acidic hydrogens and counter cation have been omitted for clarity. | ||
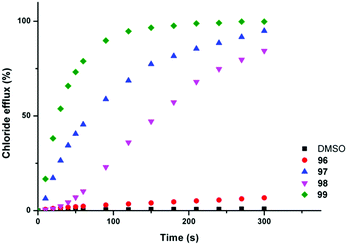
Chloride/nitrate antiport was demonstrated in a series of experiments with the fluorinated systems showing enhanced transport abilities as compared to the non-fluorinated analogues (Fig. 21 and Table 3). Presumably this enhancement is due in part to the enhanced lipophilicity of the derivatives with trifluoromethyl-substituents (Table 3). Similar results were obtained for chloride/bicarbonate exchange. Cell studies showed that the fluorinated compounds could de-acidify acidic organelles within melanoma (A375) cells and could reduce the viability of a range of human cancer cell lines in particular GLC4 (small cell human lung cancer) whilst the non-fluorinated compounds only had a limited effect on cell viability.
clog![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pa Pa |
EC50,270sb (Cl−/NO3−) | n (Cl−/NO3−) | EC50,270sb (Cl−/HCO3−) | n (Cl−/HCO3−) | |
|---|---|---|---|---|---|
a clog![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P calculated using Fieldview 2.0.2 for Macintosh (Wildman–Crippen model).
b EC50,270s defined as concentration (mol% carrier with respect to lipid) needed to obtain 50% efflux after 270 s.
c Hill coefficient.
d Accurate Hill analysis could not be performed due to low activity.
e Some of the observed activity is from H+/Cl− co-transport. P calculated using Fieldview 2.0.2 for Macintosh (Wildman–Crippen model).
b EC50,270s defined as concentration (mol% carrier with respect to lipid) needed to obtain 50% efflux after 270 s.
c Hill coefficient.
d Accurate Hill analysis could not be performed due to low activity.
e Some of the observed activity is from H+/Cl− co-transport.
|
|||||
| 96 | 3.87 | ||||
| 97 | 4.03 | 0.029 | 0.8 | 0.18 | 0.7 |
| 98 | 6.23 | 0.47 | 1.3 | 1.2 | 2.0 |
| 99 | 6.40 | 0.016e | 1.7e | 0.081e | 1.5e |
A. P. Davis, Gale and co-workers investigated how the structure of a series of thioureas with similar log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P values affects their transport properties.27 In order to do this it was necessary to find transporters that resided solely within the lipid bilayer membrane and were not partitioned between the aqueous and lipid phases (Fig. 22). A preliminary study of compounds 100a, 100b and 100e (R2 = C6H13; R1 = H, C2H5, C5H11; Ctotal 6, 8 and 11 respectively) was conducted using lucigenin containing POPC/cholesterol vesicles with the transporter present in the membrane. The vesicles contain sodium nitrate and are suspended in sodium chloride solution. Transport experiments were conducted with suspensions of vesicles at 0.4 mM, 0.2 mM and 0.1 mM lipid concentration. If the transporter is partitioned between the two phases, its activity will depend on lipid concentration. However if it is residing within the lipid bilayer solely then transport activity should be independent of concentration. It was found that this was true for compound 100e and so a total of 11 aliphatic carbons on the thiourea were necessary to confine the transporter to the lipid bilayer. A transport study was then conducted on compounds 100c, 100d, 100e, 100f and 100g which all contain 11 aliphatic carbons. It was found that compound 100e had the highest transport activity with the activities of the other compounds falling off as the thiourea group gets closer to either end of the molecule. The authors refer to this effect as lipophilic balance and attribute it to the extra stability of the transporter–chloride complexes in which the thiourea is at the end of the molecule essentially forming an amphiphile that is more stable at the lipid:water interface than complexes in which the anion binding site is in the centre of the molecule.
P values affects their transport properties.27 In order to do this it was necessary to find transporters that resided solely within the lipid bilayer membrane and were not partitioned between the aqueous and lipid phases (Fig. 22). A preliminary study of compounds 100a, 100b and 100e (R2 = C6H13; R1 = H, C2H5, C5H11; Ctotal 6, 8 and 11 respectively) was conducted using lucigenin containing POPC/cholesterol vesicles with the transporter present in the membrane. The vesicles contain sodium nitrate and are suspended in sodium chloride solution. Transport experiments were conducted with suspensions of vesicles at 0.4 mM, 0.2 mM and 0.1 mM lipid concentration. If the transporter is partitioned between the two phases, its activity will depend on lipid concentration. However if it is residing within the lipid bilayer solely then transport activity should be independent of concentration. It was found that this was true for compound 100e and so a total of 11 aliphatic carbons on the thiourea were necessary to confine the transporter to the lipid bilayer. A transport study was then conducted on compounds 100c, 100d, 100e, 100f and 100g which all contain 11 aliphatic carbons. It was found that compound 100e had the highest transport activity with the activities of the other compounds falling off as the thiourea group gets closer to either end of the molecule. The authors refer to this effect as lipophilic balance and attribute it to the extra stability of the transporter–chloride complexes in which the thiourea is at the end of the molecule essentially forming an amphiphile that is more stable at the lipid:water interface than complexes in which the anion binding site is in the centre of the molecule.
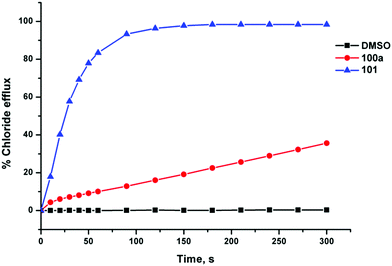
Gale and co-workers have synthesised a series of acylthioureas 101–104 (Fig. 23) and studied the anion transport ability of these compounds.28 In the absence of an anionic guest these compounds may form an intramolecular hydrogen bond (Fig. 24) which may affect their ability to transport anions. The anion transport properties of parent compound 101 were compared with thiourea analogue 100a in a chloride/nitrate antiport study (Fig. 25). The acylthiourea was found to have significantly enhanced transport properties compared its thiourea analogue (Table 4) with HPLC retention time experiments on a C18 reverse phase column revealing the enhanced lipophilicity of these compounds compared to their thiourea analogues. The authors attribute this enhancement to a lower propensity of the intramolecularly hydrogen bonded compounds to interact with water.
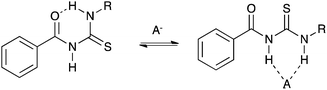 | ||
| Fig. 24 Competition between intramolecular hydrogen bond formation and anion binding in acylthiourea receptors. | ||
| Receptor | Retention time/min | Cl−/NO3−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a a |
Cl−/HCO3−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b b |
Error | n | ||
|---|---|---|---|---|---|---|---|
| EC50 | Error | n | EC50 | ||||
| a Transport data is the average of a minimum of 3 repeated Hill plot experiments. The error given is the standard deviation. b Transport data is the result of a single Hill plot analysis (therefore no errors are given). c Activity dependent on stock concentration of receptor solution-data gathered using only a 5 mM solution. | |||||||
| 100a | 4.9 | 2.7 | ±0.6 | 0.90 | 3.1 | ±0.10 | ±0.6 |
| 101 | 8.9 | 0.33 | ±0.06 | 2.07 | 0.27 | ±0.27 | ±0.78 |
| 102 | 10.5 | 0.69 | ±0.01 | 2.76 | 8.1 | ±0.24 | ±0.77 |
| 103 | 9.4 | 0.28 | ±0.02 | 2.03 | 0.28 | ±0.11 | ±0.91 |
| 104 | 12.9 | 1.1c | ±0.1c | 2.3c | 6.5c | ±0.21c | ±1.5c |
A. P. Davis and co-workers have used lipophilic scaffolds such as cholic acid to construct very effective anion transporters. Recently this group has expanded the palette of scaffolds available with reports of the use of trans-decalins29 and cyclohexane.30
Transdecalins appended with two urea groups 105a–105i (Fig. 26) were prepared and their anion transport properties studied by chloride transport into vesicles containing NaNO3 (225 mM) and lucigenin.29 The results revealed that compound 105i (presumably the most lipophilic) was the most powerful transporter from this series and the data compared to cholapod 106 (Fig. 27). Compound 105i was shown to function in transporter to lipid ratios as low as 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250
250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 (Fig. 27c).
000 (Fig. 27c).
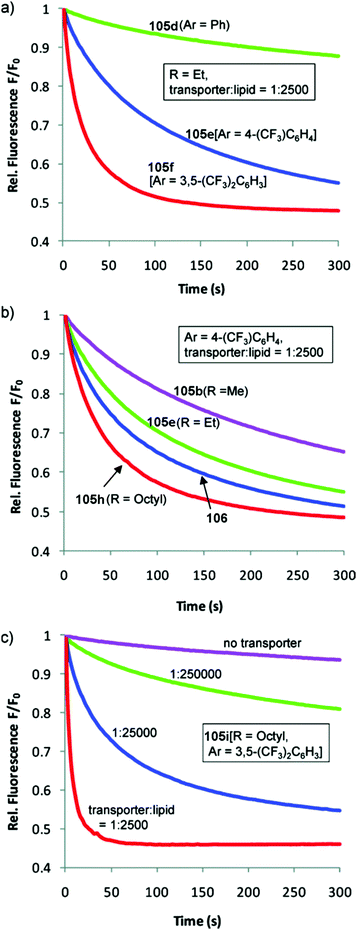 | ||
Fig. 27 Chloride transport by decalins 105a–i into vesicles containing NaNO3 (225 mM) and lucigenin: (a) varying Ar (R = Et, transporter/lipid = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2500); (b) varying R (Ar = 4-trifluoromethylphenyl, transporter/lipid = 1 2500); (b) varying R (Ar = 4-trifluoromethylphenyl, transporter/lipid = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2500). Data for cholapod 106 are also shown. (c) Varying the transporter 2500). Data for cholapod 106 are also shown. (c) Varying the transporter![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) lipid ratio for 105i, the most powerful of the transporters studied. Reproduced from ref. 29. Copyright 2011, American Chemical Society. lipid ratio for 105i, the most powerful of the transporters studied. Reproduced from ref. 29. Copyright 2011, American Chemical Society. | ||
A. P. Davis and co-workers have also developed families of transporters using a cyclohexane scaffold.30 Two families were prepared (107 and 108 – Fig. 28). Both families consist of a cyclohexane ring that has three urea or thiourea groups in axial positions linked by a methylene spacer. In 108 each spacer is functionalised with two methyl groups which act to favour the axial/in arrangement of the urea/thiourea groups and also compress the binding site so shortening potential hydrogen bonding interactions to within optimal ranges from crystallography. Proton NMR titration methods in DMSO-d6/0.5% water were used to determine the affinity of these compounds for chloride. The results (Table 5) show moderate affinities with the methylated series 108 having higher affinities than the unmethylated compounds 107. Chloride/nitrate exchange using the same lucigenin method was employed to study the transdecalin systems and the results compared against those obtained for cholapod 109. It was found that the most active compound from the cyclohexanes, compound 108e, is three times more active than cholapod 109 despite the compounds having similar sizes and calculated log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P values (and compound 108e having significantly lower affinity for chloride) and is amongst the most active compounds synthesised in Davis's group (with transport easily detectable at 1
P values (and compound 108e having significantly lower affinity for chloride) and is amongst the most active compounds synthesised in Davis's group (with transport easily detectable at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500
500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 carrier to lipid concentrations). The authors attribute the effectiveness of this series of compounds to the increased flexibility which whilst lowering anion affinity accelerates binding kinetics.
000 carrier to lipid concentrations). The authors attribute the effectiveness of this series of compounds to the increased flexibility which whilst lowering anion affinity accelerates binding kinetics.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) lipid 1
lipid 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2500)b for the families of compounds 107 and 108 based on cyclohexane
2500)b for the families of compounds 107 and 108 based on cyclohexane
| Compound | K a (M−1) | I [s−1] | t 1/2 [s] |
|---|---|---|---|
a To n-Bu4N+Cl− in DMSO-d6/0.5% water measured by 1H NMR titration. T = 298 K.
b In 200 nm vesicles formed from POPC/cholesterol (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) measured by following the decay of lucigenin fluorescence F.
c Initial rate of relative fluorescence change, F/F0.
d Decay half-life of F/F0.
e Not measured due to low solubility.
f Fluorescence decay indistinguishable from background. 3) measured by following the decay of lucigenin fluorescence F.
c Initial rate of relative fluorescence change, F/F0.
d Decay half-life of F/F0.
e Not measured due to low solubility.
f Fluorescence decay indistinguishable from background.
|
|||
| 107a | 27 | ||
| 107b | 57 | ||
| 107c | 68 | 0.00091 | 440 |
| 107d | 79 | 0.0014 | 340 |
| 107e | 99 | 0.0046 | 120 |
| 108a | 180 | ||
| 108b | 390 | 0.036 | 16 |
| 108c | 400 | 0.029 | 22 |
| 108d | 480 | 0.01 | 66 |
| 108e | 670 | 0.065 | 9 |
| 108f | 340 | 0.0017 | 190 |
| 109 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
0.093 | 6 |
Another simple scaffold – a single phenyl ring – has been employed by Gale and co-workers to prepare a variety of 1,2-bisurea compounds which are particularly effective anion transporters31 and are discussed below in terms of their biological activity.
J. T. Davis and co-workers have shown that the natural product monoacylglycerol 110a functions as a chloride/nitrate anion antiporter (Fig. 29).32 The group first looked at substitution of glycerol unit and found that in lucigenin chloride/nitrate exchange in egg-yolk phosphatidylcholine (EYPC) vesicles compound 110b promoted chloride influx whilst compounds 111 and 112 proved to be inactive so demonstrating that the 1,2-diol unit is needed for transport. The group then designed compounds 113a and 114 that may have augmented anion binding transport properties compared to parent compound 110a. These compounds contain an additional hydrogen bond donor and in the case of 114 a fluorinated chain both of which are expected to enhance the compounds' anion transport properties based on previous studies. The results (Table 6) show that the fluorinated compound 114 is almost 20 times better at facilitating chloride/nitrate antiport than parent compound 110a.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P values for monoacylglycerol analogues 110a, 113a and 114
P values for monoacylglycerol analogues 110a, 113a and 114
| Ester 110a | Amide 113aa | Perfluoro 114 | |
|---|---|---|---|
| a Hill analysis of the C18 amide 113a showed that the length of the acyl chain in 113a (C8) vs.113b (C18) had little influence on the transport efficiency, as 113b had an EC50 value of 0.076 ± 0.004. | |||
| K a (M−1) CDCl3 | 92 ± 1.7 | 1474 ± 32 | — |
| K a (M−1) CD3CN | — | 137 ± 1.8 | 193 ± 7.6 |
| EC50 270 s (mM) | 0.195 ± 0.01 | 0.078 ± 0.002 | 0.011 ± 0.002 |
| Hill coefficient | 1.45 ± 0.17 | 1.12 ± 0.045 | 0.83 ± 0.20 |
clog![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P P |
1.64 | 0.97 | 2.99 |
Method development
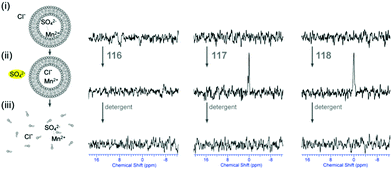
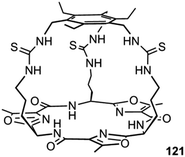
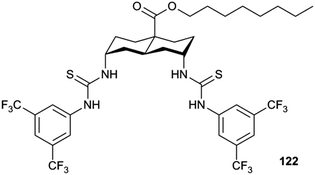
Plavec, Jolliffe, Gale and co-workers have developed a new NMR assay for sulfate transport.33 Sulfate is often used in anion transport experiments as its high energy of hydration (ΔGhyd = −1080 kJ mol−1) was believed to prevent the anion being transported from an aqueous phase into a lipid bilayer. Commonly in bicarbonate/chloride exchange experiments vesicles containing sodium chloride would be suspended in sodium sulfate solution and a pulse of sodium bicarbonate added in the extra-vesicular solution to initiate chloride/bicarbonate exchange. Prior to the addition of bicarbonate no chloride transport should be observed in the presence of a transporter, as sulfate back transport would not occur to balance the charge gradient created by chloride efflux. It was during a series of these experiments with tren-based trisureas and thioureas26 (Fig. 30) that Gale and co-workers noticed that in some cases there would be significant efflux of chloride prior to addition of bicarbonate. This occurred particularly with the pentafluorophenyl thiourea 118 and to a lesser extent the pentafluorophenyl urea 117. An NMR assay was developed in which 33S labelled sodium sulfate was encapsulated within vesicles together with paramagnetic Mn2+ cations which broaden the NMR resonance of the 33S and the vesicles suspended in sodium chloride solution (Fig. 31). Upon addition of a transporter capable of mediating chloride/sulfate exchange, the 33S NMR resonance should appear as sulfate is transported out of the intravesicular solution containing paramagnetic Mn2+. At the end of the experiment detergent was added to lyse the vesicles allowing the sulfate to again interact with the manganese ions and so the NMR resonance should be lost. The results (Fig. 31) for the tren-based compounds showed that whilst the unfluorinated compound 80 could not transport sulfate, both pentafluorophenyl derivatives 117 and 118 could. This was the first direct evidence of transmembrane sulfate transport and really a remarkable finding that such simple molecules could facilitate the transport of this highly hydrated anion. Further studies with some more complex and preorganised sulfate receptors from Jolliffe's group e.g. compound 121 (Fig. 32) showed that this compound could also mediate chloride/sulfate exchange thus demonstrating the generality of this process.Kros, Davis and co-workers have developed a method to directly visualise and quantify transport in giant unilamellar vesicles (GUVs).34 This method employs GUVs that can be visualised using light microscopy. A version of the lucigenin assay for chloride transport is used in which sodium nitrate and lucigenin are encapsulated within the GUVs composed of 70% POPC/30% cholesterol in which a transporter such as compound 122 (Fig. 33) is pre-incorporated which are suspended is sodium nitrate solution. A pulse of sodium chloride to the extra-vesicular solution initiates transport which can be visualised using confocal fluorescence microscopy. This allowed the fluorescence of individual vesicles to be tracked and hence transport events in individual vesicles to be monitored.
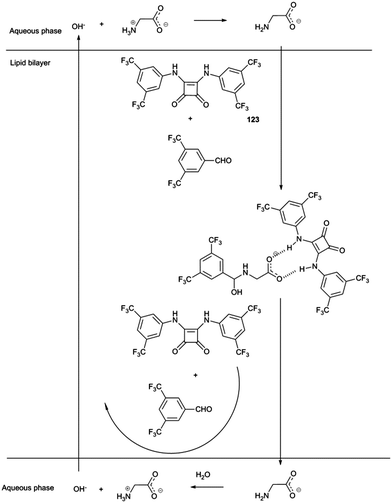
Gale and co-workers have employed dynamic covalent chemistry to facilitate the transport of amino acids across lipid bilayers.35 Squaramides had previously been shown to facilitate chloride/nitrate and chloride/bicarbonate transport across POPC lipid bilayers. Here a combination of squaramide 123 and 3,5-bistrifluoromethylbenzaldehyde were shown to facilitate the transport of glycine by both non-covalent bond formation between the squaramide and carboxylate group and reaction between the amine and the aldehyde to form either a hemi-aminal or an imine. The resulting three component assembly is significantly more lipophilic than glycine and showed an enhanced rate of transport across the bilayer (Scheme 1). This was monitored using a calcein–copper complex encapsulated within the vesicle. Influx of glycine would result in sequestration of the copper from the calcein and regeneration of the calcein fluorescence. The proposed mechanism also involves the squaramide facilitating the transport of hydroxide across the lipid bilayer. It was not until the following year that the mechanism by which hydrogen bond donor anion transport dissipate pH gradients was elucidated more fully (see below).36
Biological activity
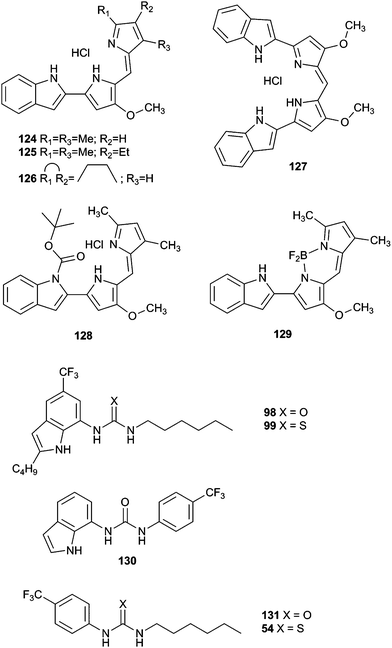
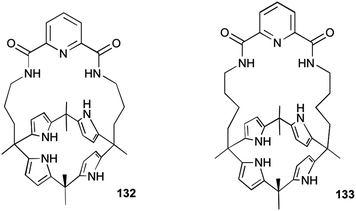
In principle, molecules capable of facilitating transmembrane transport of anions could impact ion homeostasis of cells. This is an essential parameter involved in numerous cellular processes and thus anion selective ionophores could display a range of useful biological activities. Early studies linked the transmembrane transport activity of natural products such as prodigiosin with its cytotoxicity.37 In this regard, Obatoclax, a synthetic prodiginine which has demonstrated its potential as ananticancer drug in the clinic, was studied by Quesada and co-workers.38 Obatoclax 124 and related analogues (Fig. 34) were proven to be potent nitrate, bicarbonate and chloride exchangers in model liposomes. The cytotoxicity of these compounds was assayed in the small-cell lung carcinoma cell line GLC4 and was found to correlate well with their level of transmembrane transport activity. Remarkably, the BODIPY analogue 129, inactive as anion transporter, was shown to be essentially non-toxic. This finding further support the role played by the anion transport activity in the biological activity exerted by obatoclax and related prodiginines. This correlation between transmembrane anion transport activity and cytotoxicity was also reported by Gale and co-workers in different series of synthetic molecules. Thus, tripodal, tren-based, tris(thio)ureas 118 and 120 bearing fluorinated aryl substituents were shown to display cytotoxicity toward several cancer cell lines which correlated with their anion transport activity.39 Similar results were observed for simpler compounds inspired by these tripodal ureas.26 Thus, indole-based compounds were shown to induce cytotoxicity. Fluorination was proved to be a general strategy to improve the activity and cytotoxicity of small molecule anion transport agents. IC50 values of 6–23 μM on GLC4, A375 cancerous cell lines were found for these derivatives. These values were significantly lower than the cytotoxicity exerted in the non-cancerous cell line MCF10A.The activity of calix[4]pyrrole-strapped diamides 132 and 133 was studied in a panel of cell lines by Gale, Sessler and Shin.40 These compounds were shown to mediate both chloride and sodium influx as well as to induce apoptosis via a caspase-dependent pathway. The caspase activation is related to an enhancement of cellular ROS levels, and the release of cytochrome c from the mitochondria (Fig. 35).
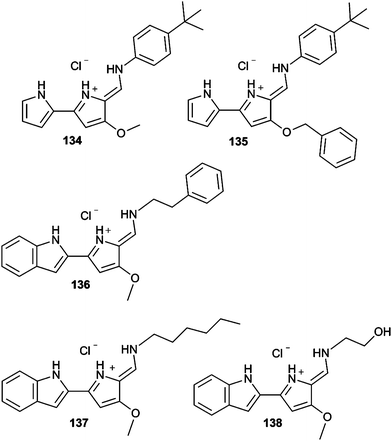
Tambjamine alkaloids represent models to explore the relationships between facilitated anion transport and the cytotoxicity exerted by these compounds because their synthetic accessibility.21 Following the study of the cytotoxicity of these compounds, a detailed study of the anti-cancer effect of tambjamine analogues 134 and 135 was carried out in several lung cancer cell lines by Pérez-Tomás and co-workers.41 Treatment of A549 cells with these derivatives led to cytoplasmic vacuolization as a result of loss of mitochondrial potential and mitochondrial swelling. This could also trigger mitophagy (mitochondrial autophagy). Indeed, accumulation of LC3II protein indicated that autophagy was being triggered by the treatment with these tambjamine analogues. On the other hand, lysosome alkalinization results in autophagy blockage and accumulation of autophagosomes and autolysosomes. All these processes led to cellular stress, resulting in activation of p38 Mitogen-Activated Protein Kinase (MAPK) in A549 cells in a dose–response manner. This signal has been reported to activate apoptosis and small activation of apoptotic markers was observed. The fact that cell viability was not recovered when a pan-caspase inhibitor (10 l M Z-VAD-FMK) was added before treatment with these derivatives suggested that although apoptosis is being triggered by these tambjamine analogues, this process is not entirely responsible for the cytotoxic effect induced by these compounds, and necrosis plays an important role as cell death mechanism. The related synthetic tambjamine analogues 137–139 were tested against lung cancer stem cells (CSC).42 This cancer cell population is noted because of its resistance to chemotherapy and its involvement in tumour recurrence and metastasis. CSC are characterised by a depolarised membrane and facilitated anion transport could impact the membrane polarisation state. Using a fluorescence assay based on a FRET of genetically encoded transmembrane proteins, it was demonstrated that compounds 137 and 138 hyperpolarised the membrane. Remarkably this effect was not observed for compound 139. Thus compound 139 resulted an appropriate negative control, being structurally very similar yet inactive as a transporter. Compounds 137 and 138 proved highly active against lung cancer CSC, inducing differentiation and elimination of CSC (Fig. 36).
Disruption of autophagy was also reported for synthetic anion transporters containing squaramide moieties. In a detailed study, Shin, Gale, Sessler and colleagues demonstrated that squaramide-based transporter 18 raised lysosomal pH.43 This pH is not suitable for lysosome enzymatic function, leading to autophagy impairment. In addition, an increase of chloride and sodium cytosolic concentrations facilitated by 18 lead to cytochrome c release from the mitochondria into the cytosol, promoting caspase-dependent apoptosis.
Another important area of research focus in the use of these compounds as protein mimics. For instance, in the case of dysfunction of natural transmembrane transport mechanisms, anion selective transporters could restore the missing activity representing a valuable therapeutic approach for related conditions, notably cystic fibrosis (CF). However, perturbation of pH gradients within cells has been linked to apoptosis – an undesirable outcome when using a compound to replace the function of a faulty ion channel.
The mechanism by which neutral hydrogen bond donating anion transporters can dissipate pH gradients across lipid bilayer membranes has been elucidated by Gale and co-workers.36 They found that depending on the acidity of the transporter, more acidic compounds could function as weak acid protonophores, more weakly acidic compounds may transport hydroxide or alternatively transport fatty acid carboxylate groups through the membrane to the other side where they can be protonated and then diffuse back across the lipid bilayer as the carboxylic acid.44 Gale also developed assays to measure the Cl−vs. H+ selectivity of transporters finding that encapsulating binding sites (such as that found in compound 139 conferred a degree of selectivity for chloride transport) but that most hydrogen bond donor anion transporters tested are unselective. Compound 139 was tested in human cancer cells and in epithelial cells. It was found not to perturb lysosomal pH gradients in the former but to transport chloride through the cell membranes in the latter. Further studies with encapsulated binding sites within calix[4]pyrrole macrocycles 4–6 showed that these compounds also facilitate selective chloride transport with a degree of selectivity remaining even in the presence of significant quantities of fatty acids.35 Gale and co-workers also measured the Cl−vs. F− selectivity of these compounds using a fluoride selective electrode to monitor fluoride transport. This group found that calix[4]pyrroles 4 and 5 with the shorter straps were selective for fluoride over chloride transport (Fig. 37).45
Another area of interest is the biocide activity of anion selective transmembrane transporters. Indeed, a number of natural ionophores (which are cation selective) found applications as antibiotics and anticoccidiostates.46 Membrane permeabilization to anions should have similar effects on these organisms yet this is an understudied area of research.
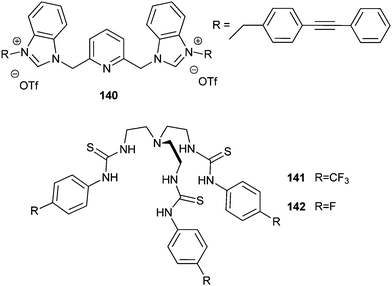
Smitzer and co-workers reported the benzimidazolium salt 140 (Fig. 38), which was identified as a chloride transporter using the lucigenin fluorescence assay with an EC50 of 0.46 mol%.47 The antibacterial activity of this benzimidazolium salt was thus evaluated in the presence of Gram-negative E. coli and Gram-positive B. thuringiensis strain. MICs of 25 μM for E. coli DH5-α, 10 μM for E. coli SK037 and 2 μM for B. thuringiensis were found. The antimicrobial activity of related, less potent chloride carriers was found to be considerably lower. Compound 140 was found to disrupt the integrity and the potential of the bacterial membranes, and this is likely the origin of its antibacterial activity. Remarkably, the low toxicity against eukaryotic cells as well as low haemolytic activity bodes well for the future development of this class of compounds as antimicrobial agents.
The inhibition of the growth of S. aureus in vitro by anion transporters was demonstrated by Francesconi, Gale, Roelens, Sessler and co-workers. Importantly, this activity was also demonstrated against methicillin-resistant Staphylococcus aureus (MRSA) strains, Mu50 and HP117. Thus, potent tris-thiourea tren-based transporters bearing fluorinated substituent such as 118, 141 and 142 (Fig. 38), were found to have MIC in the low micromolar range (0.93–1.78 mg mL−1 for the Mu50 S. aureus).48
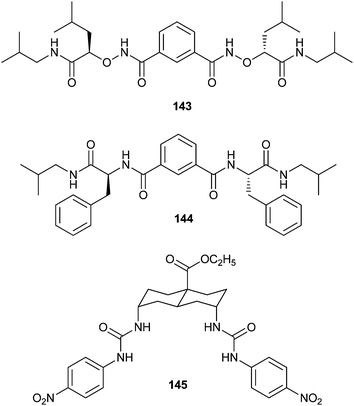
Yang and co-workers have focused their studies on isophthalamide-like small molecules. Compound 143 was found to mediate chloride transport in both liposomes and live cells.49 Using whole cell patch clamp recordings in HEK293 cells 143 was found to form anion selective channels with an anion transport activity following the order NO3− > I− > Br− > Cl− > F−. To investigate the ability of compound 143 to restore chloride permeability in cells, whole cell currents were measured in the presence of this compound for cells derived from normal human bronchial epithelia and bronchial epithelia of CF patients. These experiments revealed that this compound increase the chloride permeability of CF airway epithelial cells. This increase was found to be larger than that induced by forskolin in normal human epithelial cells, suggesting an alternative mechanism for chloride transport.
The isophthalamide-like molecules were found to mediate chloride and bicarbonate transport in liposomes.50 Patch clamp experiments indicated the formation of functional chloride channels by 144. The ability of this compound to augment chloride permeability in Calu-3 and CFBE41o (with homozygous F508 mutation) epithelial monolayers was tested with the Ussing chamber based short-circuit method. In both cases this compound was found to induce chloride dependent short circuit current increases.
Anion transport in living cells can also be monitored by fluorescence methods. For instance, FRT cells stably expressing the halide sensor YFP-H148Q/I152L (YFP-FRT cells) can be used to monitor halide transport without using specialist electrophysiological apparatus.51 Using this fluorescence based method Davis, Sheppard and co-workers screened a wide range of synthetic anion transporters.52 From these studies, the bis-ureiododecalin 145 was identified as a promising candidate, facilitating iodide transport efficiently. Moreover, this compound is able to incorporate to cell membranes readily and at effective dosages did not showed significant toxicity. Further electrophysiological studies confirmed these results. Chloride currents in YFP-FRT epithelia were measured using the Ussing chamber technique. A robust increase in ICl apical was observed. For comparison purposes, chloride current intensities in MDCK epithelia were also measured after forskolin stimulation. The maximum current induced was found to be roughly half that of natural CFTR measured for the MDCK epithelia (Fig. 39).
Non-classical transporters
Most synthetic anion transporters rely on standard hydrogen bond donors such as amide or urea NH groups as to bind anions for their subsequent transport across the bilayer membrane. Below, we describe some of the most important papers that have appeared since 2010 that use non-classical interactions to achieve transmembrane transport of anions.Over the past decade, CH hydrogen bonds have become increasingly recognized to be an important structural motif in supramolecular chemistry, nanochemistry and molecular biology. Thus, some CH donors, such as those that occur in the triazole group, form relatively strong CH hydrogen bonds. In recent years, the use of CH hydrogen bonds as a design element for the development of selective anion transporters has taken hold. Below, we describe two such leading studies wherein CH hydrogen bonds were used to develop transmembrane anion transporters. The first example of a CH hydrogen-bonding transporter was reported in 2014 by Jiang, Hou and colleagues.53 They described a pre-organized aryltriazole foldamer 146 that can bind anions using an array of C–H hydrogen bond donors (Fig. 40). Four intramolecular NH⋯N hydrogen bonds between amides and triazoles on its periphery fix 146 into a conformation that points five C–H bonds into a crescent-shaped cleft. The authors reasoned that such a pre-organized structure should favor the anion binding needed for transmembrane transport. The proposed conformation of 146 was confirmed by X-ray crystallography and also in solution using NOEs. The authors prepared a key control, as compound 147 lacks two of the intramolecular hydrogen-bonds that rigidify 146. Indeed, 147 is conformationally flexible as NOEs indicated free rotation about the single bonds between triazoles and neighboring aromatic rings. NMR titrations in CD2Cl2 showed that fully pre-organized receptor 146 had a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding constant for TBA+ Cl− (757 M−1) that was 8-fold stronger than that for the partly pre-organized receptor 147 (91 M−1). These binding studies showed that use of all possible C–H⋯anion interactions in the binding pocket relies on pre-organization of foldamer 146 by hydrogen-bond array on the receptor's periphery. The transmembrane Cl− transport activity of the two compounds was measured with EYPC LUVs using the classic lucigenin fluorescence assay, in the presence of catalytic amounts of the K+-carrier valinomycin. Valinomycin enables a K+ uniport process that helps the anionophore (146 or 147) facilitate Cl− uniport in an overall K+/Cl− symport process. Foldamer 146 was a better Cl− transporter than control 147. Thus, addition of 146 (0.75% relative to EYPC) and valinomycin (0.002%) to a LUV suspension led to a 97% increase in lucigenin fluorescence in the 12 min experiment, whereas the same amount of compound 147 increased the fluorescence by just 39% over the same time. Other transport experiments revealed that foldamer 146 functioned as a mobile carrier, had a respectable EC50 value of 0.09% relative to EYPC lipid and had a Hill coefficient (n = 1.3) indicative of a unimolecular Cl− transporter. This study demonstrated that conformational control of aryltriazole foldamers such as 146 can be used to bind and transport Cl− using CH⋯anion hydrogen-bonds. The authors concluded with the suggestion that switching elements might be incorporated so as to modulate foldamer conformation, and enable the reversible uptake and release of anions.
1 binding constant for TBA+ Cl− (757 M−1) that was 8-fold stronger than that for the partly pre-organized receptor 147 (91 M−1). These binding studies showed that use of all possible C–H⋯anion interactions in the binding pocket relies on pre-organization of foldamer 146 by hydrogen-bond array on the receptor's periphery. The transmembrane Cl− transport activity of the two compounds was measured with EYPC LUVs using the classic lucigenin fluorescence assay, in the presence of catalytic amounts of the K+-carrier valinomycin. Valinomycin enables a K+ uniport process that helps the anionophore (146 or 147) facilitate Cl− uniport in an overall K+/Cl− symport process. Foldamer 146 was a better Cl− transporter than control 147. Thus, addition of 146 (0.75% relative to EYPC) and valinomycin (0.002%) to a LUV suspension led to a 97% increase in lucigenin fluorescence in the 12 min experiment, whereas the same amount of compound 147 increased the fluorescence by just 39% over the same time. Other transport experiments revealed that foldamer 146 functioned as a mobile carrier, had a respectable EC50 value of 0.09% relative to EYPC lipid and had a Hill coefficient (n = 1.3) indicative of a unimolecular Cl− transporter. This study demonstrated that conformational control of aryltriazole foldamers such as 146 can be used to bind and transport Cl− using CH⋯anion hydrogen-bonds. The authors concluded with the suggestion that switching elements might be incorporated so as to modulate foldamer conformation, and enable the reversible uptake and release of anions.
Recently the Pittelkow and A. P. Davis groups reported together on the anion transport properties of lipophilic biotin[6]urils, macrocyclic receptors that use only CH hydrogen bonds to bind anions within their central cavity (Fig. 41).54 Both 1H NMR and ITC titrations revealed that the biotin methyl ester 148 has a ∼100-fold selectivity for binding Cl−vs. HCO3− in CD3CN. The authors noted that HCO3− and NO3−, anions with similar shapes but different basicities, had similar affinities for receptor 148. They attributed this result to the unique character of the CH⋯anion hydrogen-bond, which has much less of an electrostatic component than conventional hydrogen-bonds. Butyl ester 149 was a particularly effective transmembrane anion transporter, as judged by the classical “lucigenin assay” in POPC:cholesterol liposomes. Lipophilicity was an important feature, since the butyl ester 149 was a better transporter than the ethyl or methyl esters. Butyl ester 149 was a relatively potent Cl− transporter, as it showed a t1/2 = 180 s at the low carrier/lipid loading of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2500. Notably, 149 was highly selective for transporting Cl−vs. HCO3− in this liposome model. Thus, while 149 catalyzed efficient Cl−/NO3− transmembrane it hardly promoted any observable Cl−/HCO3− exchange over background. The authors noted that this high degree of Cl−/HCO3− selectivity for biotin[6]uril 149, which uses only CH hydrogen-bonds to coordinate the target anion, was in marked contrast with receptors that use conventional hydrogen-bond donors such as urea and amide NH groups. They attributed this selectivity to the relatively “soft” nature of CH as a hydrogen bond donor, which would favour binding of more hydrophobic anions, such as Cl− and NO3−, over the more basic HCO3−. The Cl−/HCO3− selectivity shown by biotin[6]uril ester 149 is potentially important for biological applications that require specific Cl− transport vs. HCO3− transport.
2500. Notably, 149 was highly selective for transporting Cl−vs. HCO3− in this liposome model. Thus, while 149 catalyzed efficient Cl−/NO3− transmembrane it hardly promoted any observable Cl−/HCO3− exchange over background. The authors noted that this high degree of Cl−/HCO3− selectivity for biotin[6]uril 149, which uses only CH hydrogen-bonds to coordinate the target anion, was in marked contrast with receptors that use conventional hydrogen-bond donors such as urea and amide NH groups. They attributed this selectivity to the relatively “soft” nature of CH as a hydrogen bond donor, which would favour binding of more hydrophobic anions, such as Cl− and NO3−, over the more basic HCO3−. The Cl−/HCO3− selectivity shown by biotin[6]uril ester 149 is potentially important for biological applications that require specific Cl− transport vs. HCO3− transport.
 | ||
| Fig. 41 Macrocyclic biotin[6]uril esters use CH⋯anion hydrogen-bonds to selectively transport Cl− across lipid membranes. (a) Structures of biotin[6]uril esters 148 and 149. The lipophilic butyl ester 149 is a better anion transporter than methyl ester 148. (b) An energy-minimized model of the biotin[6]uril macrocycle's core showing the CH⋯Cl− hydrogen-bonds. Reproduced from ref. 54 which is published under a CC-BY license. | ||
The anion–π interaction, an attractive force between an anion and an electron-deficient aromatic π system, is a noncovalent bond finding increasing use in the construction of functional supramolecular systems. To bind anions with aromatic surfaces a compound's quadrupole moment has to be positive instead of negative. This reversal in quadrupole moment is accomplished by substituting the aromatic ring with strong electron-withdrawing groups. In earlier work, Matile and colleagues had shown that oligomers of π-acidic naphthalenediimide (NDI), dubbed “anion–π slides”, catalyzed anion transport across lipid bilayers, presumably by enabling anions to hop from one π-acidic site to the next as they move across the bilayer.55 Although these early studies on the rigid-rods suggested that anion–π interactions were responsible for transport, these authors sought more convincing evidence for the functional relevance of these non-classical interactions.
To address the fundamental question of whether anion–π interactions are responsible for transmembrane anion transport, Matile and colleagues “deconstructed” the NDI rods to give a family of simpler NDI models 150–152.56 This work has become a landmark in the field, as both experiment and computation demonstrated that non-classical interactions can be used to effect transmembrane anion transport. First, the authors used mass spectrometry to measure relative affinities of anions binding to 150–152; competition experiments revealed a Cl− binding selectivity of 152 ≫ 151 > 150. These results showed that increasing the NDI's π acidity, along with minimizing steric congestion near the anion binding site, provided a receptor 152 with optimal anion binding affinity. The Cl− anion transport activity of NDI monomers 150–152 was tested in EYPC liposomes using the standard HPTS fluorescence assay and compound 152 was found to be the most active of the compounds, showing significant Cl− transport activity even at nM concentrations (EC50 = 330 nM). Computational models of NDI 152, an analogue with 2 electron-withdrawing cyano groups, showed Cl− binding to the π-acidic surface and stabilized by C–H⋯anion hydrogen-bonds with a peripheral phenyl group. The major accomplishment in this study was to correlate the strength of the non-covalent anion–π interaction for a particular compound with its efficiency as a transmembrane anion transporter. Thus, the higher a compound's affinity for binding anions then the better was its anion transport activity, supporting the hypothesis that it is indeed the anion–π interactions that account for the anion-transport function displayed by the rigid rods in Matile's earlier papers, and by the π-acidic monomers 150–152 described in this present study (Fig. 42).
Halogen bonding, the non-covalent interaction between an electron-deficient halogen and a neutral or anionic Lewis base, is being increasingly used in supramolecular chemistry, catalysis and, now, in anion transport. Matile and colleagues prepared a series of ditopic calix[4]arene receptors, including 153–155, with different halogen substitution patterns on the calixarene's lower rim. These ion-pair receptors selectively bind tetramethylammonium (TMA+) cations within the calixarene's upper rim, while the lower rim coordinates chloride anion. This study's major goal was to use these calix[4]arenes to systematically evaluate the contributions of various non-classical interactions, including CH⋯anion hydrogen bonds, anion–π interactions and anion–halogen bonds to the transmembrane transport of anions.57 Using their standard HPTS assays, which monitors intravesicular pH, the authors demonstrated that calixarenes 153–155 catalyze transmembrane Cl−/OH− exchange with varying efficiencies. Calixarene 153, with its pentafluoroaryl groups, proved to be the best Cl− transporter in the series, with a relatively low EC50 value of 25 μM. DFT models of the 153·TMACl complex indicated a binding energy of −58.3 kcal mol−1 and revealed a structure wherein 3 of the 4 pentafluoroaryl groups in 153 formed anion–π interactions with bound Cl−. Significantly, a single-atom F to I mutation of 153 to give the m-iodo analogue 154 resulted in near complete loss of Cl− transport activity (EC50 for 154 = 1000 μM). The authors concluded that this drop-off in anion transport ability was because this m-iodo analogue 154 uses a combination of anion–π and anion–halogen interactions to actually bind Cl− too strongly to allow effective anion transport. In support of this hypothesis, 19F NMR titrations showed that receptor 154 (but not active transporters 153 or 155) binds TBACl in acetone-d6. Moreover, DFT calculations indicated a greater binding energy of −70.9 kcal mol−1 for the 154·TMACl complex as compared to that for the 153·TMACl. The authors reasoned that weakening Cl− binding by removing the anion–π interactions, while keeping the anion–halogen bonds, should restore transmembrane Cl− transport by analogue 155. Indeed, replacing all the –F atoms in 154 with –H atoms, gave an analogue 155 with an EC50 value of 32 μM, more than 30 times lower than that of 154. Presumably, it is the anion–halogen bonds in 155 that enable functional transport across lipid bilayers (Fig. 43).
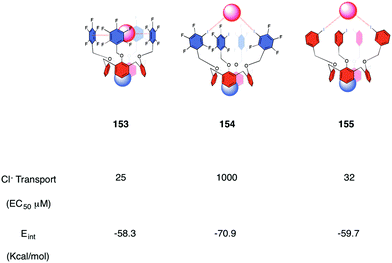 | ||
| Fig. 43 Summary of anion transport data and DFT calculated energies to Cl-anion for calixarenes 153–155. | ||
In another nice demonstration of the importance of anion–π interactions, Ballester, Matile and colleagues reported that calix[4]pyrrole derivatives 156–158, containing aryl substituents at two opposing meso-positions promoted selective transmembrane transport of nitrate over other anions (Fig. 44).58 Using NMR titration data they determined the strength of the nitrate–π interaction as a function of the meso-substituent and found that the calix[4]pyrroles’ affinity for nitrate correlated well with the electrostatic surface potential for a particular aryl group. The 3,5-dinitrophenyl analogue 158, the most π-acidic compound in the series, had the highest binding constant for nitrate. An X-ray structure of the complex TBA+[158·NO3]− showed the nitrate anion bound between the two opposing meso-aryl walls and oriented perpendicular to the rings. The ability of calix[4]pyrrole derivatives to transport anions across EYPC LUVs was evaluated using the standard HPTS assay. All calix[4]pyrroles tested showed Cl− transport ability, but those analogues with the most π-acidic surfaces, namely 157 and 158, were the best Cl− transporters. Importantly, they also showed transport selectivity for the trigonal planar NO3− anion vs. Cl−. This remarkable nitrate transport selectivity by 157 and 158 was attributed to (1) the specific nitrate–π interactions implied by the X-ray crystal structure and (2) the ability of the π-acidic walls to effectively sequester nitrate as it is moved across the hydrophobic lipid bilayer by the calix[4]pyrrole carrier.
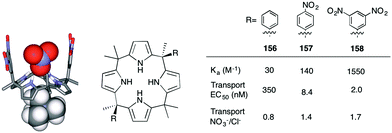 | ||
| Fig. 44 Left: X-ray crystal structure of 158·NO3− complex. Right: Summary of anion binding and transport data for calix[4]pyrroles 156–158. | ||
It is not just structurally complex carriers, such as calixarenes and calixpyrroles, that can use non-classical interactions as a driving force for anion transport. In an interesting study, Matile and colleagues showed that low-molecular weight compounds, such as 159 (and even a 1-carbon carrier 160) with halogen bond acceptors could function as transmembrane anion transporters.59 DFT calculations revealed that an individual Cl− could be encased in a hydrophobic cocoon by six molecules of the perfluoroiodide 160, thus facilitating the membrane transport process by protecting the charged anion from the non-polar lipid environment. HPTS fluorescence assays revealed a remarkably low value of EC50 = 21.6 μM for 160, with a Hill coefficient of n = 4.7, which is consistent with the DFT calculations for formation of halogen bonded capsules. Although trifluoroiodomethane 160 is a relatively poor anion transporter, it is remarkable that it works at all since it contains just a single carbon. Finally, one attractive feature of anion carriers such as 160 is their innate hydrophobicity and complete lack of hydrophilic hydrogen bonding groups, which can cause aggregation or encounter dehydration penalties when entering a lipophilic environment (Fig. 45).
Much like halogen bonds, chalcogen bonds form upon electron donation from a Lewis base into the Lewis acidic σ hole found in electron-deficient sulfur atoms. Very recently, Matile and colleagues introduced synthetic anion transporters that use chalcogen bonds to coordinate anions.60 Electron-deficient dithieno [3,2-b;2′,3′-d]thiophenes (DTTs) were shown to chelate anions using the Lewis acidic σ holes on two adjacent sulfur atoms that were located in a constrained binding site. Both the strength of anion binding in solution and the rates of anion transport across lipid bilayers increased with the increasing electron deficiency of the sulfur acceptor atoms in a series of DTT anionophores. The best Cl− anion transporter was compound 161 (EC50 = 1.9 μM), whose σ holes on the sulfur atoms were enhanced by substitution of the thiophene rings with electron-withdrawing cyano groups and a sulfoxide unit. The authors noted that DTT ligands such as 161, which use non-classical chalcogen bonds to coordinate and transport anions, complement the 2,2′-bipyrrole unit, a truly classic hydrogen bonding motif for anion binding and transport (Fig. 46).
Conclusions
In the last few years there has been a rapid progress in the development of anion selective transmembrane transporters. Highly efficient systems both inspired in naturally occurring derivatives and de novo designed systems have appeared. Important insights into transport mechanism and transporter selectivity have been obtained. Importantly, supramolecular transmembrane anion transport is making the leap from model systems to cells. Early studies show great promise. A range of anion transporters that can dissipate pH gradients have been prepared and shown reduce the viability of human cancer cell lines. Recent experiments show that one mechanism that may be responsible for this is disruption of autophagy. Meanwhile compounds that are selective chloride transporters are beginning to be developed and shown to transport chloride through epithelial cell membranes. Compounds that transport chloride but do not perturb pH gradients within cells may be candidates to replace the function of faulty anion channels in diseases like cystic fibrosis. The chasm between studies in model membranes and applications of anion transporters in biological systems has been bridged and a new application in supramolecular medicinal chemistry is in sight.Acknowledgements
PAG thanks the University of Sydney and the Australian Research Council (DP170100118) for funding. JTD thanks the Office of Basic Energy Sciences, U.S. Department of Energy (DE-FG02-98ER14888) for funding. RQ thanks Consejería de Educación de la Junta de Castilla y León (BU340U13 and BU092U16) and La Marató de TV3 Foundation (20132730) for funding.References
- (a) D. C. Gadsby, Nat. Rev. Mol. Cell Biol., 2009, 10, 344–352 CrossRef CAS PubMed; (b) B. Hille, Ion Channels of Excitable Membranes, Sinauer Associates, Sunderland, MA, 3rd edn, 2001 Search PubMed.
- (a) F. M. Ashcroft, Nature, 2006, 440, 440–447 CrossRef CAS PubMed; (b) E. Marban, Nature, 2002, 415, 213–218 CrossRef CAS PubMed; (c) M. J. Ackerman and D. E. Clapham, N. Engl. J. Med., 1997, 336, 1575–1586 CrossRef CAS PubMed.
- (a) F. Ratjen, S. C. Bell, S. M. Rowe, C. H. Goss, A. L. Quittner and A. Bush, Nat. Rev. Dis. Primers, 2015, 1, 15010 CrossRef PubMed; (b) D. C. Gadsby, P. Vergani and L. Csanady, Nature, 2006, 440, 477–483 CrossRef CAS PubMed; (c) M. J. Welsh and A. E. Smith, Cell, 1993, 73, 1251–1254 CrossRef CAS PubMed; (d) J. R. Riordan, J. M. Rommens, B. S. Kerem, N. Alon, R. Rozmahel, Z. Grzelczak, J. Zielenski, S. Lok, N. Plavsic, J. L. Chou, M. L. Drumm, M. C. Iannuzzi, F. S. Collins and L. C. Tsui, Science, 1989, 245, 1066–1073 CAS; (e) P. M. Quinton, Nature, 1983, 301, 421–422 CrossRef CAS PubMed.
- F. M. Ashcroft, Ion Channels and Disease, Academic Press, 2000 Search PubMed.
- D. K. Smith, J. Chem. Educ., 2005, 82, 393–400 CrossRef CAS . For recent developments in anion receptor chemistry including transmembrane anion transport in cells see: P. A. Gale, E. N. W. Howe and X. Wu, Chem, 2016, 1, 351–422 Search PubMed.
- P. A. Gale, C. C. Tong, C. J. Haynes, O. Adeosun, D. E. Gross, E. Karnas, E. M. Sedenberg, R. Quesada and J. L. Sessler, J. Am. Chem. Soc., 2010, 132, 3240–3241 CrossRef CAS PubMed.
- M. Yano, C. C. Tong, M. E. Light, F. P. Schmidtchen and P. A. Gale, Org. Biomol. Chem., 2010, 8, 4356–4363 CAS.
- I. W. Park, J. Yoo, B. Kim, S. Adhikari, S. Kuk Kim, Y. Yeon, C. J. E. Haynes, J. L. Sutton, C. C. Tong, V. M. Lynch, J. L. Sessler, P. A. Gale and C. Lee, Chem. – Eur. J., 2012, 18, 2514–2523 CrossRef CAS PubMed.
- C. J. E. Haynes, S. J. Moore, J. R. Hiscock, I. Marques, P. J. Costa, V. Félix and P. A. Gale, Chem. Sci., 2012, 3, 1436–1444 RSC.
- N. Busschaert, I. L. Kirby, S. Young, S. J. Coles, P. N. Horton, M. E. Light and P. A. Gale, Angew. Chem., Int. Ed., 2012, 51, 4426–4430 CrossRef CAS PubMed.
- R. B. P. Elmes, N. Busschaert, D. D. Czech, P. A. Gale and K. A. Jolliffe, Chem. Commun., 2015, 51, 10107–10110 RSC.
- E. N. W. Howe, N. Busschaert, X. Wu, S. N. Berry, J. Ho, M. E. Light, D. D. Czech, H. A. Klein, J. A. Kitchen and P. A. Gale, J. Am. Chem. Soc., 2016, 138, 8301–8308 CrossRef CAS PubMed.
- S. J. Edwards, H. Valkenier, N. Busschaert, P. A. Gale and A. P. Davis, Angew. Chem., 2015, 127, 4675–4679 CrossRef.
- B. A. McNally, A. V. Koulov, B. D. Smith, J. B. Joos and A. P. Davis, Chem. Commun., 2005, 1087–1089 RSC.
- C. R. Yamnitz, S. Negin, I. A. Carasel, R. K. Winter and G. W. Gokel, Chem. Commun., 2010, 46, 2838–2840 RSC.
- X. Wei, G. Zhang, Y. Shen, Y. Zhong, R. Liu, N. Yang, F. Y. Almkhaizim, M. A. Kline, L. He, M. Li, Z.-L. Lu, Z. Shao and B. Gong, J. Am. Chem. Soc., 2016, 138, 2749–2754 CrossRef CAS PubMed.
- T. Saha, S. Dasari, D. Tewari, A. Prathap, K. M. Sureshan, A. K. Bera, A. Mukerjee and P. Talukdar, J. Am. Chem. Soc., 2014, 136, 14128–14135 CrossRef CAS PubMed.
- T. Saha, A. Gautam, A. Mukerjee, M. Lahiri and P. Talukdar, J. Am. Chem. Soc., 2016, 138, 16443–16451 CrossRef CAS PubMed.
- A. Vargas Jentzsch and S. Matile, J. Am. Chem. Soc., 2013, 135, 5302–5303 CrossRef CAS PubMed.
- L. Chen, W. Si, G. Tang, Z.-T. Li and J.-L. Hou, J. Am. Chem. Soc., 2013, 135, 2152–2153 CrossRef CAS PubMed.
- V. Saggiomo, S. Otto, I. Marques, V. Félix, T. Torroba and R. Quesada, Chem. Commun., 2012, 48, 5274–5276 RSC.
- N. Busschaert, S. J. Bradberry, M. Wenzel, C. J. E. Haynes, J. R. Hiscock, I. L. Kirby, L. E. Karagiannidis, S. J. Moore, N. J. Wells, J. Herniman, G. J. Langley, P. N. Horton, M. E. Light, I. Marques, P. J. Costa, V. Félix, J. G. Frey and P. A. Gale, Chem. Sci., 2013, 4, 3036–3045 RSC.
- M. J. Spooner and P. A. Gale, Chem. Commun., 2015, 51, 4883–4886 RSC.
- N. Busschaert, P. A. Gale, C. J. E. Haynes, M. E. Light, S. J. Moore, C. C. Tong, J. T. Davis and W. A. Harrell Jr., Chem. Commun., 2010, 46, 6252–6254 RSC.
- N. J. Andrews, C. J. E. Haynes, M. E. Light, S. J. Moore, C. C. Tong, J. T. Davis, W. A. Harrell Jr. and P. A. Gale, Chem. Sci., 2011, 2, 256–260 RSC.
- N. Busschaert, M. Wenzel, M. E. Light, P. Iglesias-Hernández, R. Pérez-Tomás and P. A. Gale, J. Am. Chem. Soc., 2011, 133, 14136–14148 CrossRef CAS PubMed.
- H. Valkenier, C. J. E. Haynes, J. Herniman, P. A. Gale and A. P. Davis, Chem. Sci., 2014, 5, 1128–1134 RSC.
- C. J. E. Haynes, N. Busschaert, I. L. Kirby, J. Herniman, M. E. Light, N. J. Wells, I. Marques, V. Félix and P. A. Gale, Org. Biomol. Chem., 2014, 12, 62–72 CAS.
- S. Hussain, P. R. Brotherhood, L. W. Judd and A. P. Davis, J. Am. Chem. Soc., 2011, 133, 1614–1617 CrossRef CAS PubMed.
- J. A. Cooper, S. T. G. Street and A. P. Davis, Angew. Chem., Int. Ed., 2014, 53, 5609–5613 CrossRef CAS PubMed.
- S. J. Moore, M. Wenzel, M. E. Light, R. Morley, S. J. Bradberry, P. Gómez-Iglesias, V. Soto-Cerrato, R. Pérez-Tomás and P. A. Gale, Chem. Sci., 2012, 3, 2501–2509 RSC.
- S. Bahmanjah, N. Zhang and J. T. Davis, Chem. Commun., 2012, 48, 4432–4434 RSC.
- N. Busschaert, L. E. Karagiannidis, M. Wenzel, C. J. E. Haynes, N. J. Wells, P. G. Young, D. Makuc, J. Plavec, K. A. Jolliffe and P. A. Gale, Chem. Sci., 2014, 5, 1118–1127 RSC.
- H. Valkenier, N. López Mora, A. Kros and A. P. Davis, Angew. Chem., Int. Ed., 2015, 54, 2137–2141 CrossRef CAS PubMed.
- X. Wu, N. Busschaert, N. J. Wells, Y.-B. Jiang and P. A. Gale, J. Am. Chem. Soc., 2015, 137, 1476–1484 CrossRef CAS PubMed.
- X. Wu, L. W. Judd, E. N. W. Howe, A. M. Withecombe, V. Soto-Cerrato, H. Li, N. Busschaert, H. Valkenier, R. Perez-Tomas, D. N. Sheppard, Y.-B. Jiang, A. P. Davis and P. A. Gale, Chem, 2016, 1, 127–146 CAS.
- J. L. Sessler, L. R. Eller, W.-S. Cho, S. Nicolaou, A. Aguilar, J. T. Lee, V. M. Lynch and D. J. Magda, Angew. Chem., Int. Ed., 2005, 44, 5989–5992 CrossRef CAS PubMed.
- B. D. de Greñu, P. I. Hernandez, M. Espona, D. Quiñonero, M. E. Light, T. Torroba, R. Pérez-Tomás and R. Quesada, Chem. – Eur. J., 2011, 17, 14074–14083 CrossRef PubMed.
- N. Busschaert, M. Wenzel, M. E. Light, P. Iglesias-Hernández, R. Pérez-Tomás and P. A. Gale, J. Am. Chem. Soc., 2011, 133, 14136–14148 CrossRef CAS PubMed.
- S. Ko, S. K. Kim, A. Share, V. M. Lynch, J. Park, W. Namkung, W. Van Rossom, N. Busschaert, P. A. Gale, J. L. Sessler and I. Shin, Nat. Chem., 2014, 6, 885–892 CrossRef CAS PubMed.
- A. M. Rodilla, L. Korrodi-Gregório, E. Hernando, P. Manuel-Manresa, R. Quesada, R. Pérez-Tomás and V. Soto-Cerrato, Biochem. Pharmacol., 2017, 126, 23–33 CrossRef CAS PubMed.
- V. Soto-Cerrato, P. Manuel-Manresa, E. Hernando, S. Calabuig-Fariñas, A. Martinez-Romero, V. Fernández-Dueñas, K. Sahlholm, T. Knopfel, M. Garcia-Valverde, A. M. Rodilla, E. Jantus-Lewintre, R. Farras, F. Ciruela, R. Perez-Tomas and R. Quesada, J. Am. Chem. Soc., 2015, 137, 15892–15898 CrossRef CAS PubMed.
- N. Busschaert, S.-H. Park, K.-H. Baek, Y. P. Choi, J. Park, E. N. W. Howe, J. R. Hiscock, L. E. Karagiannidis, I. Marques, V. Félix, W. Namkung, J. L. Sessler, P. A. Gale and I. Shin, Nat. Chem., 2017 DOI:10.1038/NCHEM.2706.
- X. Wu and P. A. Gale, J. Am. Chem. Soc., 2016, 138, 16508–16514 CrossRef CAS PubMed.
- H. J. Clarke, E. N. W. Howe, X. Wu, F. Sommer, M. Yano, M. E. Light, S. Kubik and P. A. Gale, J. Am. Chem. Soc., 2016, 138, 16515–16522 CrossRef CAS PubMed.
- I. Alfonso and R. Quesada, Chem. Sci., 2013, 4, 3009–3019 RSC.
- C. R. Elie, G. David and A. R. Schmitzer, J. Med. Chem., 2015, 58, 2358–2366 CrossRef CAS PubMed.
- A. I. Share, K. Patel, C. Nativi, E. J. Cho, O. Francesconi, N. Busschaert, P. A. Gale, S. Roelens and J. L. Sessler, Chem. Commun., 2016, 52, 7560–7563 RSC.
- B. Shen, X. Li, F. Wang, X. Yao and D. Yang, PLoS One, 2012, 7, e34694 CAS.
- P.-Y. Liu, S.-T. Li, F.-F. Shen, W.-H. Ko, X.-Q. Yao and D. Yang, Chem. Commun., 2016, 52, 7380–7383 RSC.
- L. J. V. Galietta, P. M. Haggie and A. S. Verkman, FEBS Lett., 2001, 499, 220–224 CrossRef CAS PubMed.
- H. Li, H. Valkenier, L. W. Judd, P. R. Brotherhood, S. Hussain, J. A. Cooper, O. Jurček, H. A. Sparkes, D. N. Sheppard and A. P. Davis, Nat. Chem., 2016, 8, 24–32 CrossRef CAS PubMed.
- J. Shang, W. Si, Y. Che, J.-L. Hou and H. Jiang, Org. Lett., 2014, 16, 4008–4011 CrossRef CAS PubMed.
- M. Lisbjerg, H. Valkenier, B. M. Jessen, H. Al-Kerdi, A. P. Davis and M. Pittelkow, J. Am. Chem. Soc., 2015, 137, 4948–4951 CrossRef CAS PubMed.
- J. Mareda and S. Matile, Chem. – Eur. J., 2009, 15, 28–37 CrossRef CAS PubMed.
- R. E. Dawson, A. Hennig, D. P. Weimann, D. Emery, V. Ravikumar, J. Montenegro, T. Takeuchi, S. Gabutti, M. Mayor, J. Mareda, C. A. Schalley and S. Matile, Nat. Chem., 2010, 2, 533–538 CrossRef CAS PubMed.
- A. Vargas Jentzsch, D. Emery, J. Mareda, P. Metrangolo, G. Resnati and S. Matile, Angew. Chem., Int. Ed., 2011, 50, 11675–11678 CrossRef CAS PubMed.
- L. Adriaenssens, C. Estarella, A. Vargas Jentzsch, M. M. Belmonte, S. Matile and P. Ballester, J. Am. Chem. Soc., 2013, 135, 8324–8330 CrossRef CAS PubMed.
- A. Vargas Jentzsch, D. Emery, J. Mareda, S. K. Nayak, P. P. Metrangolo, G. Resnati, N. Sakai and S. Matile, Nat. Commun., 2012, 3, 905–912 CrossRef PubMed.
- S. Benz, M. Macchione, Q. Verolet, J. Mareda, N. Sakai and S. Matile, J. Am. Chem. Soc., 2016, 138, 9093–9096 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2017 |